

Chinese Journal of Organic Chemistry >
Research Progress on Light-Promoted Transition Metal-Catalyzed C-Heteroatom Bond Coupling Reactions
Received date: 2022-02-15
Revised date: 2022-05-05
Online published: 2022-06-01
Supported by
National Natural Science Foundation of China(21871171)
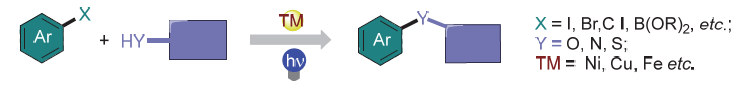
The photo-transition metal synergistically catalyzed coupling reaction provides a new research strategy for the development of metal-catalyzed coupling reactions. In this catalytic system, the excited state photosensitizer controls the valence state of the transition metal intermediate through a single electron transfer process, there by regulating the coupling reaction process, especially for the process that is difficult to occur in traditional transition metal catalysis. In addition, the excited photosensitizers could also promote the coupling reaction through the energy transfer process. Simultaneously, the light-promoted transition metal-catalyzed C—X bond coupling reactions without external photosensitizers have also been rapidly developed. Light and transition metals synergistically catalyzed coupling reactions provide an important tool for the construction of C-heteroatom bonds, showcasing broad application prospects in synthetic chemistry.
Geyang Song , Dong Xue . Research Progress on Light-Promoted Transition Metal-Catalyzed C-Heteroatom Bond Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2022 , 42(8) : 2275 -2299 . DOI: 10.6023/cjoc202202018
| [1] | (a) Hartwig, J. F. Nature 2008, 455, 314. |
| [1] | (b) Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954. |
| [1] | (c) Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599. |
| [1] | (d) Castillo, P. R.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564. |
| [2] | Crabtree, R. H. The Organometallic Chemistry of the Transitionmetals, John Wiley & Sons, 2009. |
| [3] | (a) Kalthoff, F. S.; James, M. J.; Teders, M.; Pitzer, L.; Glorius, F. Chem. Soc. Rev. 2018, 47, 7190. |
| [3] | (b) Zhou, Q.; Zou, Y.; Lu, L.; Xiao, W. Angew. Chem., Int. Ed., 2019, 58, 1586. |
| [4] | Kalthoff, F. S.; James, M. J.; Teders, M.; Pitzer, L.; Glorius, F. Chem. Soc. Rev. 2018, 47, 7190. |
| [5] | Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898. |
| [6] | Arias-Rotondoa, D. M.; McCusker, J. K. Chem. Soc. Rev. 2016, 45, 5803. |
| [7] | (a) Chen, J; Cen, J.; Xu, X.; Li, X. Catal. Sci. Technol. 2016, 6, 349. |
| [7] | (b) Lang, X., Chen, X.; Zhao, J. Chem. Soc. Rev. 2014, 43, 473. |
| [7] | (c) Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Green Chem. 2016, 18, 5391. |
| [7] | (d) Savateev, A.; Ghosh, I.; König, B.; Antonietti, M. Angew. Chem., Int. Ed. 2018, 57, 15936. |
| [8] | Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075. |
| [9] | (a) Hopkinson, M. N.; Sahoo, B.; Li, J.; Glorius, F. Chem.-Eur. J. 2014, 20, 3874. |
| [9] | (b) Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035. |
| [10] | (a) Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C. Nat. Rev. Chem. 2017, 1, 0052. |
| [10] | (b) Milligan, J. A.; Phelan, J. P.; Badir, S. O.; Molander, G. A. Angew. Chem., Int. Ed. 2019, 58, 6152. |
| [11] | (a) PrierDanica, C. K.; Rankic, A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [11] | (b) Cheung, K. P. S.; Sarkar, S.; Gevorgyan, V. Chem. Rev. 2022, 122, 1543. |
| [12] | Cavedon, C.; Seeberger, P. H.; Pieber, B. Eur. J. Org. Chem. 2020, 1379. |
| [13] | (a) Pitsinos, E. N.; Vidali, V. P.; Couladouros, E. A. Eur. J. Org. Chem. 2011, 2011, 1207. |
| [13] | (b) Nicolaou, K. C.; Boddy, C. N. C.; Brase, S.; Winssinger, N. Angew. Chem., Int. Ed. 1999, 38, 2096. |
| [13] | (c) Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283. |
| [13] | (d) Brown, D. G.; Bostro?m, J. J. Med. Chem. 2016, 59, 4443. |
| [14] | (a) Bariwal, J.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 9283. |
| [14] | (b) Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564. |
| [14] | (c) Forero-Cortés, P. A.; Haydl, A. M. Org. Process Res. Dev. 2019, 23, 1478. |
| [14] | (d) Dorel, R.; C. Grugel, P.; Haydl, A. M. Angew. Chem., Int. Ed. 2019, 58, 17118. |
| [15] | (a) Ullmann, F. Chem. Ber. 1903, 36, 2382. |
| [15] | (b) Goldberg, I. Chem. Ber. 1906. 39, 1691. |
| [15] | (c) Kosugi, M.; Kameyama, M.; Migita, T. Chem. Lett. 1983, 12, 927. |
| [15] | for reviews, see: (d) Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. |
| [15] | (e) Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525. |
| [16] | (a) Qiao, J.; Lam, P. Y. S. Synthesis 2011, 829. |
| [16] | (b) West, M. J.; Fyfe, J. W. B.; Vantourout, J. C.; Watson, A. J. B. Chem. Rev. 2019, 119, 12491. |
| [17] | (a) Levin, M. D.; Kim, S.; Toste, F. D. ACS Cent. Sci. 2016, 2, 293. |
| [17] | (b) Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C. Nat. Rev. Chem. 2017, 1, 0052. |
| [17] | (c) Chan, A. Y.; Perry, I. B.; Bissonnette, N. B.; Buksh, B. F.; Edwards, G. A.; Frye, L. I.; Garry, O. L.; Lavagnino, M. N.; Li, B. X.; Liang, Y.; Mao, E.; Millet, A.; Oakley, J. V.; Reed, N. L.; Sakai, H. A.; Seath, C. P.; MacMillan, D. W. C. Chem. Rev. 2022, 122, 1485. |
| [18] | (a) Tasker, S. Z.; Jamison, T. F. J. Am. Chem. Soc. 2015, 137, 9531. |
| [18] | (b) Corcoran, E. B.; Pirnot, M. T.; Lin, S.; Dreher, S. D.; DiRocco, D. A.; Davies, I. W.; Buchwald, S. L; MacMillan, D. W. C. Science 2016, 353, 279. |
| [18] | (c) Oderinde, M. S.; Jones, N. H.; Juneau, A.; Frenette, M.; Aquila, B.; Tentarelli, S.; Robbins, D. W.; Johannes, J. W. Angew. Chem., Int. Ed. 2016, 55, 13219. |
| [18] | (d) Kim, T.; McCarver, S. J.; Lee, C.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2018, 57, 3488. |
| [18] | (e) Du, Y.; Pearson, R. M.; Lim, C. H.; Sartor, S. M.; Ryan, M. D.; Yang, H. S.; Damrauer, N. H.; Miyake, G. M. Chem.-Eur. J. 2017, 23, 10962. |
| [18] | (f) Huang, Z.; Xie, K.; Meng, G.; Ma, J.; Xue, D.; Yang, J. CN 108409618, 2018. |
| [18] | (g) For the nickel catalyzed C—N coupling with near UV light. Lim, C. H.; Kudisch, M.; Liu, B.; Miyake, G. M. J. Am. Chem. Soc. 2018, 140, 7667. |
| [18] | (h) Li, C.; Kawamata, Y.; Nakamura, H.; Vantourout, J. C.; Liu, Z.; Hou, Q.; Bao, D.; Starr, J. T.; Chen, J.; Yan, M.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 13088. |
| [18] | (i) Kawamata, Y.; Vantourout, J. C.; Hickey, D. P.; Bai, P.; Chen, L.; Hou, Q. L.; Qiao, W.; Barman, K.; Edwards, M. A.; Castro, A. F. G.; Gruyter, J. N.; Nakamura, H.; Knouse, K.; Qin, C.; Clay, K. J.; Bao, D.; Li, C.; Starr, J. T.; Irizarry, C. G.; Sach, N.; White, H. S.; Neurock, M.; Minteer, S. D.; Baran, P. S. J. Am. Chem. Soc. 2019, 141, 6392. |
| [18] | (j) Laudadio, G.; Barmpoutsis, E.; Schotten, C.; Struik, L.; Govaerts, S. D.; Browne, L.; Noel, T. J. Am. Chem. Soc. 2019, 141, 5664. |
| [18] | (k) Kudisch, Lim, M.; C.; Thordarson, P.; Miyake, G. M. J. Am. Chem. Soc. 2019, 141, 19479. |
| [19] | Creutz, S. E.; Lotito, K. J.; Fu, G. C.; Peters, J. C. Science 2012, 338, 647. |
| [20] | Bissember, A. C.; Lundgren, R. J.; Creutz, S. E.; Peters, J. C.; Fu, G. C. Angew. Chem., Int. Ed. 2013, 52, 5129. |
| [21] | Ziegler, D. T.; Choi, J.; Mun?oz, M. J. M.; Bissember, A. C.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2013, 135, 13107. |
| [22] | Do, H.; Bachman, S.; Bissember, A. C.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2014, 136, 2162. |
| [23] | Yoo, W.; Tsukamoto, T.; Kobayashi, S. Org. Lett. 2015, 17, 3640. |
| [24] | Tasker, S. Z.; Jamison, T. F. J. Am. Chem. Soc. 2015, 137, 9531. |
| [25] | Yoo, W.; Tsukamoto, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2015, 54, 6587. |
| [26] | Oderinde, M. S.; Jones, N. H.; Juneau, A.; Frenette, M.; Aquila, B.; Tentarelli, S.; Robbins, D. W.; Johannes, J. W. Angew. Chem., Int. Ed. 2016, 55, 13219. |
| [27] | Konev, M. O.; McTeague, T. A.; Johannes, J. W. ACS Catal. 2018, 8, 9120. |
| [28] | Li, G.; Yang, L.; Liu, J. Zhang, W.; Cao, R.; Wang, C.; Zhang, Z.; Xiao, J.; Xue, D. Angew. Chem., Int. Ed. 2021, 60, 5230. |
| [29] | Corcoran, E. B.; Pirnot, M. T.; Lin, S.; Dreher, S. D.; DiRocco, D. A.; Davies, W.; Buchwald, S. L.; MacMillan, D. W. C. Science 2016, 353, 279. |
| [30] | Till, N. A.; Tian, L.; Dong, Z.; Scholes, G. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2020, 142, 15830. |
| [31] | Du, Y.; Pearson, R. M.; Lim, C. H.; Sartor, S. M.; Ryan, M. D.; Yang, H.; Damrauer, N. H.; Miyake, G. M. Chem.-Eur. J. 2017, 23, 10962. |
| [32] | Lim, C. H.; Kudisch, M.; Liu, B.; Miyake, G. M. J. Am. Chem. Soc. 2018, 140, 7667. |
| [33] | Kudisch, M.; Lim, C. H.; Thordarson, P.; Miyake, G. M. J. Am. Chem. Soc. 2019, 141, 19479. |
| [34] | Liu, Y.; Liang, D.; Lu, L.; Xiao, W. Chem. Commun. 2019, 55, 4853. |
| [35] | Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Science 2019, 365, 36. |
| [36] | Engl, P. S.; Ha?ring, A. P.; Berger, F.; Berger, G.; Bitria?n, A. P.; Ritter, T. J. Am. Chem. Soc. 2019, 141, 13346. |
| [37] | Gisbertz, S.; Reischauer, S.; Pieber, B. Nat. Catal. 2020, 3, 611. |
| [38] | Song, G.; Yang, L.; Li, J.; Tang, W.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.; Xue, D. Angew. Chem., Int. Ed. 2021, 60, 21536. |
| [39] | Kim, T.; McCarver, S. J.; Lee, C.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2018, 57, 3488. |
| [40] | Blackburn, J. M.; Kanegusuku, A. L. G.; Scott, G. E.; Roizen, J. L. Org. Lett. 2019, 21, 7049. |
| [41] | Wu, C.; Bian, Q.; Ding, T.; Tang, M.; Zhang, W.; Xu, Y.; Liu, B.; Xu, H.; Li, H.-B.; Fu, H. ACS Catal. 2021, 11, 9561. |
| [42] | Feng, M.; Tang, B.; Liang, S.; Jiang, X. Curr. Top. Med. Chem. 2016, 16, 1200. |
| [43] | (a) Patani, G. A.; LaVoie, E. J. Chem. Rev. 1996, 96, 3147. |
| [43] | (b) Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832. |
| [44] | Boyd, D. A. Angew. Chem., Int. Ed. 2016, 55, 15486. |
| [45] | Rahate, A. S.; Nemade, K. R.; Waghuley, S. A. Rev. Chem. Eng. 2013, 29, 471. |
| [46] | (a) Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534. |
| [46] | (b) Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596. |
| [46] | (c) Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N. Angew. Chem., Int. Ed. 2017, 56, 2487. |
| [47] | (a) Kwong, F. Y.; Buchwald, S. L. Org. Lett. 2002, 4, 3517. |
| [47] | (b) Murata, M.; Buchwald, S. L. Tetrahedron 2004, 60, 7397. |
| [47] | (c) Ferna?ndez, R. M. A.; Shen, Q.; Hartwig, J. F. J. Am. Chem. Soc. 2006, 128, 2180. |
| [47] | (d) Alvaro, E.; Hartwig, J. F. J. Am. Chem. Soc. 2009, 131, 7858. |
| [47] | (e) Sayah, M.; Organ, M. G. Chem.-Eur. J. 2011, 17, 11719. |
| [47] | (f) Gogoi, P.; Hazarika, S.; Sarma, M. J.; Sarma, K.; Barman, P. Tetrahedron 2014, 70, 7484. |
| [48] | (a) Liu, B.; Lim, C.; Miyake, G. M. J. Am. Chem. Soc. 2017, 139, 13616. |
| [48] | (b) Liu, B.; Lim, C.; Miyake, G. M. Synlett 2018; 29, 2449. |
| [48] | (c) Li, G.; Yan, Q.; Gan, Z.; Li, Q.; Dou, X.; Yang, D. Org. Lett. 2019, 21, 7938. |
| [48] | (d) Guo, W.; Tao, K.; Tan, W.; Zhao, M.; Zheng, L.; Fan, X. Org. Chem. Front. 2019, 6, 2048. |
| [48] | (e) Chalotra, N.; Rizvi, M. A. B.; Shah, A. Org. Lett. 2019, 21, 4793. |
| [48] | (f) Jiang, M., Li, H.; Yang, H.; Fu, H. Angew. Chem., Int. Ed. 2017, 56, 874. |
| [48] | (g) Czyz, M. L.; Weragoda, G. K.; Monaghan, R.; Connell, T. U.; Brzozowski, M.; Scully, A. D.; Burton, J.; Lupton, D. W.; Polyzos, A. Org. Biomol. Chem. 2018, 16, 1543. |
| [49] | Wang, X.; Cuny, G. D.; Noe?l, T. Angew. Chem., Int. Ed. 2013, 52, 7860. |
| [50] | Uyeda, C.; Tan, Y.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc. 2013, 135, 9548. |
| [51] | Johnson, M. W.; Hannoun, K. I.; Tan, Y.; Fu, G.; C. Peters, J. C. Chem. Sci. 2016, 7, 4091. |
| [52] | Oderinde, M. S.; Frenette, M.; Robbins, D. W.; Aquila, B.; Johannes, J. W. J. Am. Chem. Soc. 2016, 138, 1760. |
| [53] | Jouffroy, M.; Kelly, C. B.; G. Molander, A. Org. Lett. 2016, 18, 876. |
| [54] | Liu, N.; Hofman, K.; Herbert, A.; Manolikakes, G. Org. Lett. 2018, 20, 760. |
| [55] | Yue, H.; Zhu, C.; Rueping, M. Angew. Chem., Int. Ed. 2018, 57, 1371. |
| [56] | (a) Rappoport, Z. The Chemistry of Phenols, Wiley-VCH, Weinheim, 2003. |
| [56] | (b) Tyman, J. H. P. Synthetic and Natural Phenols, Elsevier, New York, 1996. |
| [56] | (c) Arpe, H. J. Industrial Organic Chemistry, 5th ed., Wiley-VCH, Weinheim, 2010, pp. 359-374. |
| [56] | (d) Larock, R. C. Comprehensive Organic Transformations, VCH, New York, 1989, p. 966. |
| [56] | (e) Otera, J. Esterification:Methods, Reactions and Applications, Wiley, New York, 2003. |
| [56] | (f) Otera, J. Chem. Rev. 1993, 93, 1449. |
| [56] | (g) Ishihara, K. Tetrahedron 2009, 65, 1085. |
| [56] | (h) Chakraborti, A. K.; Shivani. J. Org. Chem. 2006, 71, 5785. |
| [56] | (i) Carle, M. S.; Shimokura, G. K.; Murphy, G. K. Eur. J. Org. Chem. 2016, 3930. |
| [57] | Cohen, T.; Dietz, A. G.; Miser, J. R. J. Org. Chem. 1977, 42, 2053. |
| [58] | (a) Wolter, M.; Nordmann, G.; Job, G. E.; Buchwald, S. L. Org. Lett. 2002, 4, 973. |
| [58] | (b) Li, F; Wang, Q; Ding, Z; Tao, G. Org. Lett. 2003, 5, 2169. |
| [58] | (c) Ma, D.; Cai, Q. Org. Lett. 2003, 5, 3799. |
| [58] | (d) Tlili, A.; Xia, N.; Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 8725. |
| [58] | (e) Zhao, D.; Wu, N.; Zhang, S.; Xi, P.; Su, X.; Lan, J.; You, J. Angew. Chem., Int. Ed. 2009, 48, 8729. |
| [58] | (f) Yang, D.; Fu, H. Chem. Eur. J. 2010, 16, 2366. |
| [58] | (g) Ding, G.; Han, H. Jiang, T.; Wu, T.; Han, B. Chem. Commun. 2014, 50, 9072. |
| [58] | (h) Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. J. Am. Chem. Soc. 2016, 138, 13493. |
| [58] | (i) Fier, P. S.; Maloney, K. M. Org. Lett. 2017, 19, 3033. |
| [58] | (j) Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. J. Am. Chem. Soc. 2019, 141, 3541. |
| [59] | (a) Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941. |
| [59] | (b) Chan, D. M. T.; Monaco, K. L.; Wang, R.; Winteres, M. P. Tetrahedron Lett. 1998, 39, 2933. |
| [59] | (c) Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937. |
| [59] | (d) Decicco, C. P.; Song, S.; Evans, D. A. Org. Lett. 2001, 3, 1029. |
| [59] | (e) Chan, D. M. T.; Monaco, K. L.; Li, R.; Bonne, D.; Clark, C. G.; Lam, P. Y. S. Tetrahedron Lett. 2003, 44, 3863. |
| [60] | Terrett, J. A.; Cuthbertson, J. D.; Shurtleff, V. W.; MacMillan, D. W. C. Nature 2015, 524, 330. |
| [61] | Sang, R.; Korkis, S. E.; Su, W.; Ye, F.; Engl, P. S.; Berger, F.; Ritter, T. Angew. Chem., Int. Ed. 2019, 58, 16161. |
| [62] | Yang, L.; Hu, L; Lai, C.; Li, G.; Zhang, W.; Cao, R.; Liu, F.; Wang, C.; Xiao, J.; Xue, D. Angew. Chem., Int. Ed. 2020, 59, 12714. |
| [63] | Sun, R.; Qin, Y.; Ruccolo, S.; Schnedermann, C.; Costentin, C.; Nocera, D. G. J. Am. Chem. Soc. 2019, 141, 89. |
| [64] | Zhou, Q.; Lu, F.; Liu, D; Lu, L; Xiao, W. Org. Chem. Front., 2018, 5, 3098. |
| [65] | Yang, L.; Huang, Z.; Li, G.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.; Xue, D. Angew. Chem., Int. Ed. 2018, 57, 1968. |
| [66] | (a) Khedkar, M. V.; Sasaki, T.; Bhanage, B. M. ACS Catal. 2013, 3, 287. |
| [66] | (b) Cheng, X.; Li, Y.; Su, Y. M.; Yin, F.; Wang, J.; Sheng, J.; Vora, H. U.; Wang, X.; Yu, J. J. Am. Chem. Soc. 2013, 135, 1236. |
| [66] | (c) Rosa, J. N.; Reddy, R. S.; Candeias, N. R.; Cal, P. M. S. D.; Gois, P. M. P. Org. Lett. 2010, 12, 2686. |
| [67] | (a) Takise, R.; Muto, K.; Yamaguchi, J. Chem. Soc. Rev. 2017, 46, 5864. |
| [67] | (b) Quasdorf, K. W.; Tian, X.; Garg, N. K. J. Am. Chem. Soc. 2008, 130, 14422. |
| [67] | (c) Li, B.; Li, Y.; Lu, X; Liu, J.; Guan, B.; Shi, Z. Angew. Chem., Int. Ed. 2008, 47, 10124. |
| [67] | (d) Shimasaki, T.; Tobisu, M.; Chatani, N. Angew. Chem., Int. Ed. 2010, 49, 2929. |
| [67] | (e) Takise, R.; Itami, K.; Yamaguchi, J. Org. Lett. 2016, 18, 4428. |
| [68] | Luo, F.; Pan, C.; Qian, P.; Cheng, J. Synthesis 2010, 2005. |
| [69] | (a) Zhang, L.; Zhang, G.; Zhang, M.; Cheng, J. J. Org. Chem. 2010, 75, 7472. |
| [69] | (b) Dai, J. J.; Liu, J. H.; Luo, D. F.; Liu, L. Chem. Commun. 2011, 47, 677. |
| [69] | (c) Ruso, J. S.; Rajendiran, N.; Kumaran, R. S. Tetrahedron Lett. 2014, 55, 2345. |
| [70] | (a) Petersen, T. B.; Khan, R.; Olofsson, B. Org. Lett. 2011, 13, 3462. |
| [70] | (b) Xie, H.; Yang, S.; Zhang, C.; Ding, M.; Liu, M.; Guo, J.; Zhang, F. J. Org. Chem. 2017, 82, 5250. |
| [70] | (c) Dohi, T.; Koseki, D.; Sumida, K.; Okada, K.; Mizuno, S.; Kato, A. Adv. Synth. Catal. 2017, 359, 3503. |
| [70] | (d) Bhattarai, B.; Tay, J. H.; Nagorn, P. Chem. Commun. 2015, 51, 5398. |
| [71] | Kreye, O.; Meier, M. A. R. RSC Adv. 2015, 5, 53155. |
| [72] | Welin, E. R.; Le, C.; Arias-Rotondo, D. M.; McCusker, J. K.; Mac Millan, D. W. C. Science 2017, 355, 380. |
| [73] | Lu, J.; Pattengale, B.; Liu, Q.; Yang, S.; Shi, W.; Li, S.; Huang, J.; Zhang, J. J. Am. Chem. Soc. 2018, 140, 13719. |
| [74] | Pieber, B.; Malik, J. A.; Cavedon, C.; Gisbertz, S.; Savateev, A.; Cruz, D.; Heil, T.; Zhang, G.; Seeberger, P. H. Angew. Chem., Int. Ed. 2019, 58, 9575. |
| [75] | Zhu, D.; Li, H.; Xu, Z.; Li, H.; Young, D. J.; Lang, J. Org. Chem. Front. 2019, 6, 2353. |
| [76] | Theil, F. Angew. Chem. Int. Ed. 1999, 38, 2345. |
| [77] | Hartwig, J. F. Angew. Chem. Int. Ed. 1998, 37, 2046. |
| [78] | Muci, A. R., Buchwald, S. L. Top. Curr. Chem. 2002, 219, 131. |
| [79] | Hartwig, J. F. Nature, 2008, 455, 314. |
| [80] | Enthaler, S. Chem. Soc. Rev. 2011, 40, 4912. |
| [81] | Tan, Y.; Mun?oz, M. J. M.; Fu, G. C.; Peters, J. C. Chem. Sci. 2014, 5, 2831. |
| [82] | Liu, L.; Nevado, C. Organometallics 2021, 40, 2188. |
| [83] | Zhu, D.; Jiang, S.; Wu, Q. Wang, H.; Li, H.; Li, H.-X. Org. Lett. 2021, 23, 8327. |
/
| 〈 |
|
〉 |