

Chinese Journal of Organic Chemistry >
Synthesis and Antitumor Activity of Hederagenin Derivatives
Received date: 2022-04-07
Revised date: 2022-05-25
Online published: 2022-06-09
Supported by
National Natural Science Foundation of China(81960626); National Natural Science Foundation of China(82160666); National Natural Science Foundation of China(82160628); Jilin Provincial Education Department of China(JJKH 20191156KJ)
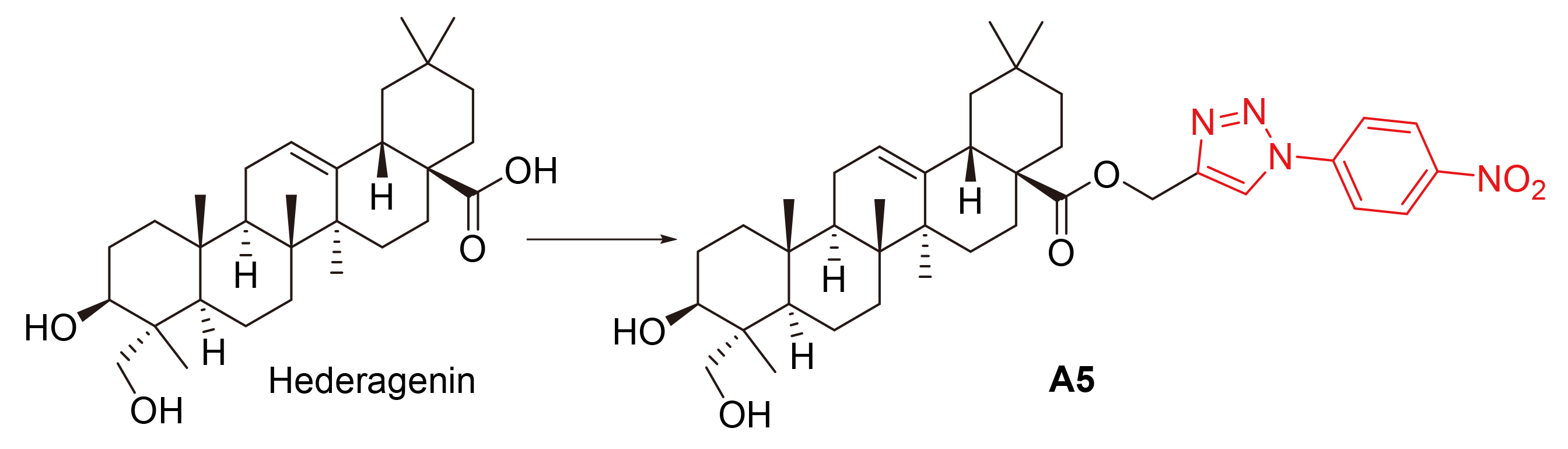
Using hederagenin as lead compound, three series of novel hederagenin derivatives containing various heterocycles were designed and synthesized. Their structures were characterized by 1H NMR, 13C NMR and HRMS. The in vitro antitumor activities of these compounds on human non-small cell lung cancer cells (A549), human breast cancer cells (MCF-7), human colon cancer cells (HCT116), human colon cancer cells (SW620), human hepatoma cells (HepG-2) and human hepatoma cells (BEL7402) were evaluated by thiazolyl blue (MTT) method. The experimental results showed that (3β,4α)-3,23-dihydroxy- olean-12-ene-28-acid-(1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl) methyl ester (A5) had the strongest anti-proliferative activity (IC50=2.05 μmol/L) on HCT116 cells. Western blotting experiments showed that compound A5 may exert its antitumor activity through NF-κB signaling pathway.
Key words: hederagenin; anticancer; thiazolyl blue (MTT) assay; Western blotting
Xing Huang , Changhao Zhang , Hao Deng , Qingkun Shen , Hongyan Guo , Zheshan Quan , Zhiyong Li , Lili Jin . Synthesis and Antitumor Activity of Hederagenin Derivatives[J]. Chinese Journal of Organic Chemistry, 2022 , 42(9) : 2877 -2887 . DOI: 10.6023/cjoc202204021
| [1] | Bonsignore, G.; Patrone, M.; Grosso, F.; Martinotti, S.; Ranzato, E. Int. J. Mol. Sci. 2021, 22, 10380. |
| [2] | Sung, H.; Siegel, R. L.; Jemal, A.; Ferlay, J.; Laversanne, M.; Soerjomataram, I.; Bray, F. Ca-Cancer J. Clin. 2021, 71, 209. |
| [3] | Boshuizen, J.; Peeper, D. S. Mol. Cell 2020, 78, 1002. |
| [4] | Choi, H.; Cho, S. Y.; Pak, H. J.; Kim, Y.; Choi, J. Y.; Lee, Y. J.; Gong, B. H.; Kang, Y. S.; Han, T.; Choi, G.; Cho, Y.; Lee, S.; Ryoo, D.; Park, H. J. Cheminf. 2017, 9, 2. |
| [5] | Sauter, E. R. Expert Rev. Clin. Pharmacol. 2020, 13, 265. |
| [6] | Wei, Y.; Ma, C. M.; Chen, D. Y.; Hattori, M. Phytochemistry (Elsevier) 2008, 69, 1875. |
| [7] | Kang, H. R.; Eom, H. J.; Lee, S. R.; Choi, S. U.; Kang, K. S.; Lee, K. R.; Kim, K. H. Nat. Prod. Commun. 2015, 10, 1929. |
| [8] | Rodriguez-Hernandez, D.; Demuner, A. J.; Barbosa, L. C. A.; Csuk, R.; Heller, L. Eur. J. Med. Chem. 2015, 105, 57. |
| [9] | Zhang, R. H.; Jin, R.; Deng, H.; Shen, Q. K.; Quan, Z. S.; Jin, C. M. Korean J. Parasitol. 2021, 59, 297. |
| [10] | Bock, V. D.; Speijer, D.; Hiemstra, H.; Van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971. |
| [11] | Liu, X. J.; Zhang, H. J.; Quan, Z. S. Med. Chem. Res. 2017, 26, 1935. |
| [12] | Zhang, G. R.; Ren, Y.; Yin, X. M.; Quan, Z. S. Lett. Drug Des. Discovery 2018, 15, 406. |
| [13] | Shao, Y. P.; Han, R. B.; Wu, H. F.; Piao, F. Y. Med. Chem. Res. 2018, 27, 642. |
| [14] | Huang, X.; Chen, T.; Han, R. B.; Piao, F. Y. CNS Neurol. Disord.: Drug Targets 2018, 17, 448. |
| [15] | Yan, G. F.; Ying, H. L.; Yang, L.; Fang, L. Y.; Xuan, Y. Y.; Jin, C. H.; Ji, Z. C.; Wang, M.; Ai, J.; Guan, P. M.; Piao, H. R.; Jin, C. M.; Jin, C. H. ChemMedChem 2021, 16, 2354. |
| [16] | Zhang, T. Y.; Li, C. S.; Cui, M. Y.; Bai, X. Q.; Chen, J. H.; Song, Z. W.; Feng, B.; Liu, X. K. Mol. Diversity 2021, 25, 861. |
| [17] | Zhang, T. Y.; Li, C. S.; Li, P.; Bai, X. Q.; Guo, S. Y.; Jin, Y.; Piao, S. J. Mol. Diversity 2022, 26, 27. |
| [18] | Pang, L.; Liu, C. Y.; Gong, G. H.; Quan, Z. S. Acta Pharm. Sin. B 2020, 10, 628. |
| [19] | Ma, Q. Q.; Bian, M.; Gong, G. H.; Bai, C. M.; Liu, C. Y.; Wei, C. X.; Quan, Z. S.; Du, H. H. J. Nat. Prod. 2022, 85, 15. |
| [20] | Liu, H. J.; Huang, X.; Shen, Q. K.; Deng, H.; Li, Z.; Quan, Z. S. Iran. J. Pharm. Res. 2021, 20, 144. |
| [21] | Guo, H. Y.; Xing, Y.; Suna, Y. Q.; Liua, C.; Xua, Q.; Shang, F. F.; Zhang, R. H.; Jin, X. J.; Lee, F. C. J. J.; Kang, D.; Shen, Q. K.; Quan, Z. S. J. Ginseng Res. 2022. |
| [22] | Shang, F. F.; Wang, M. Y.; Ai, J. P.; Shen, Q. K.; Guo, H. Y.; Jin, C. M.; Chen, F. E.; Quan, Z. S.; Jin, L.; Zhang, C. Med. Chem. Res. 2021, 30, 2228. |
| [23] | Zhang, H. B.; Shen, Q. K.; Wang, H.; Jin, C. M.; Jin, C. M.; Quan, Z. S. Eur. J. Med. Chem. 2018, 158, 414. |
| [24] | Sheng, C.; Che, X.; Wang, W.; Wang, S.; Cao, Y.; Miao, Z.; Yao, J.; Zhang, W. Eur. J. Med. Chem. 2011, 46, 5276. |
| [25] | Wei, Z. Y.; Chi, K. Q.; Wang, K. S.; Wu, J.; Liu, L. P.; Piao, H. R. Bioorg. Med. Chem. Lett. 2018, 28, 1797. |
| [26] | Chi, K. Q.; Wei, Z. Y.; Wang, K. S.; Wu, J.; Chen, W. Q.; Jin, X. J.; Piao, H. R. Bioorg. Chem. 2017, 75, 157. |
| [27] | Rashid, S.; Dar, B. A.; Majeed, R.; Hamid, A.; Bhat, B. A. Eur. J. Med. Chem. 2013, 66, 238. |
| [28] | Shen, Q. K.; Liu, C. F.; Zhang, H. J.; Tian, Y. S.; Quan, Z. S. Bioorg. Med. Chem. Lett. 2017, 27, 4871. |
| [29] | Wang, S. B.; Liu, H.; Li, G. Y.; Li, J.; Li, X. J.; Lei, K.; Wei, L. C.; Quan, Z. S.; Wang, X. K.; Liu, R. M. Pharmacol. Rep. 2019, 71, 1244. |
| [30] | Ghosh, K.; Sarkar, A. R.; Chattopadhyay, A. P. Eur. J. Org. Chem. 2012, 2012, 1311. |
| [31] | Wei, Z. Y.; Cui, B. R.; Cui, X.; Wu, Y. L.; Fu, Y.; Liu, L. P.; Piao, H. R. Chem. Biol. Drug Des. 2017, 89, 47. |
| [32] | Liu, Y. X.; Cui, Z. P.; Liu, B.; Cai, B. L.; Li, Y. H.; Wang, Q. M. J. Agric. Food Chem. 2010, 58, 2685. |
| [33] | Zhang, L. H.; Zhang, Z. H.; Li, M. Y.; Wei, Z. Y.; Jin, X. J.; Piao, H. R. Bioorg. Med. Chem. Lett. 2019, 29, 1440. |
| [34] | Lei, K. F.; Wu, Z. M.; Huang, C. H. Biosens. Bioelectron. 2015, 74, 878. |
| [35] | Liu, Y.; Li, J.; Xu, H.; Zhang, Y.; Liu, Y.; Liu, X. Int. J. Mol. Med. 2009, 24, 653. |
| [36] | Han, Z. X.; Wang, H. M.; Jiang, G.; Du, X. P.; Gao, X. Y.; Pei, D. S. Cancer Biother. Radiopharm. 2013, 28, 398. |
| [37] | Zhang, T.; Saghatelian, A. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2013, 1831, 1542. |
| [38] | Hwang, S. G.; Park, J.; Park, J. Y.; Park, C. H.; Lee, K. H.; Cho, J. W.; Hwang, J. I.; Seong, J. Y. PLoS One 2012, 7, e44259. |
| [39] | Yenmis, G.; Yaprak Sarac, E.; Besli, N.; Soydas, T.; Tastan, C.; Dilek Kancagi, D.; Yilanci, M.; Senol, K.; Karagulle, O. O.; Ekmekci, C. G.; Ovali, E.; Tuncdemir, M.; Ulutin, T.; Kanigur Sultuybek, G. Acta Histochem. 2021, 123, 151709. |
| [40] | Zerbini, L. F.; Wang, Y.; Czibere, A.; Correa, R. G.; Cho, J. Y.; Ijiri, K.; Wei, W.; Joseph, M.; Gu, X.; Grall, F.; Goldring, M. B.; Zhou, J. R.; Libermann, T. A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 13618. |
| [41] | Denlinger, C. E.; Rundall, B. K.; Jones, D. R. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 28. |
| [42] | Wang, Z.; Li, M. Y.; Mi, C.; Wang, K. S.; Ma, J.; Jin, X. Int. J. Mol. Sci. 2017, 18, 1619. |
| [43] | Bian, M.; Zhen, D.; Shen, Q. K.; Du, H. H.; Ma, Q. Q.; Quan, Z. S. Bioorg. Chem. 2021, 107, 104598. |
| [44] | Ko, J. H.; Lee, J. H.; Jung, S. H.; Lee, S. G.; Chinnathambi, A.; Alharbi, S. A.; Yang, W. M.; Um, J. Y.; Sethi, G.; Ahn, K. S. Molecules 2017, 22, 1157. |
/
| 〈 |
|
〉 |