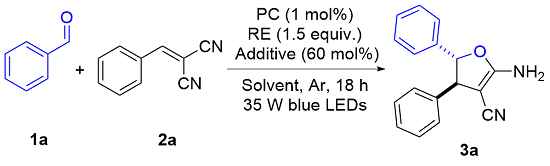
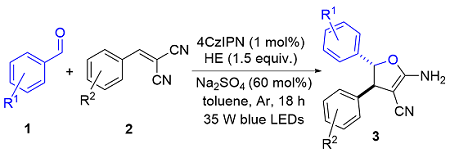
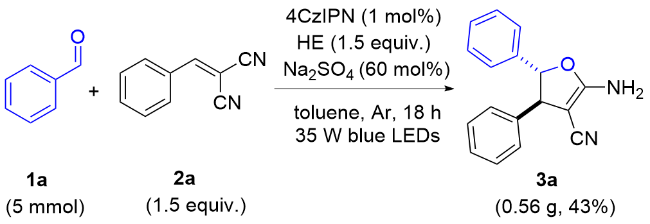
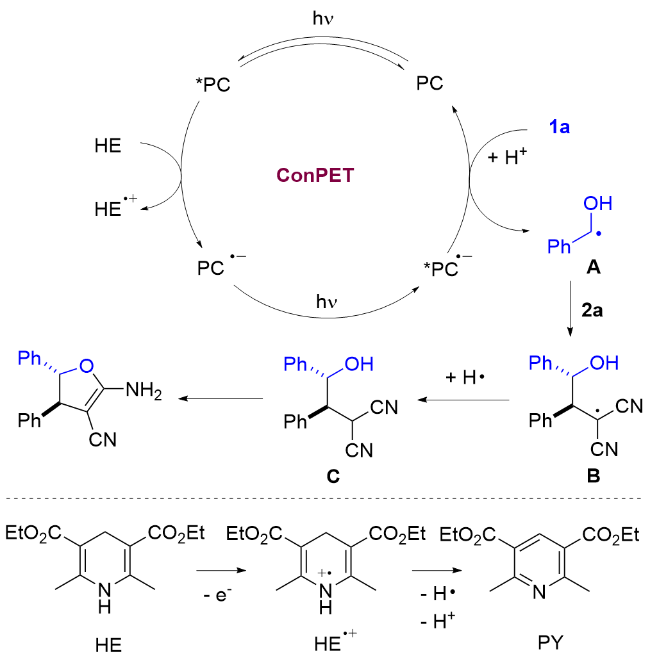
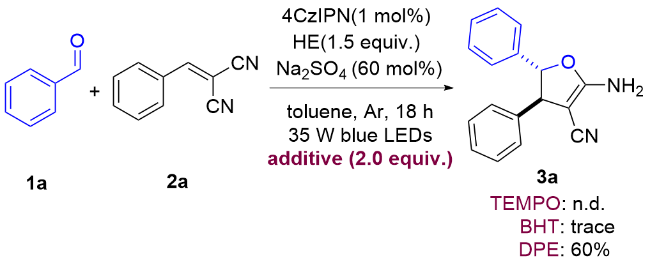以3a的合成为例, 反应在烘箱干燥的20 mL圆底反应管中进行. 称取0.2 mmol苯甲醛、0.3 mmol苄烯丙二腈、0.001 mmol 4CzIPN、0.5 mmol HE和60 mol%的硫酸钠, 加入反应管中, 并用2.0 mL甲苯溶解, 密封并通过充放气3次充入氩气, 用35 W蓝光LED灯照射, 搅拌18 h. 反应过程通过TLC监测. 反应结束后混合物经乙酸乙酯萃取、无水硫酸钠干燥、过滤及减压旋蒸得到粗产品, 再使用硅胶(200~300 目)进行柱层析或硅胶(GF254)进行薄层层析(石油醚/二氯甲烷, V∶V=4∶1~1∶1)得到产物3a. 其他部分不够纯的产物通过重结晶进一步纯化.
2-氨基-4,5-二苯基-4,5-二氢呋喃-3-甲腈(3a): 白色固体, 产率71%. m.p. 120~125 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.42 (s, 2H), 7.09~7.01 (m, 5H), 7.01~6.92 (m, 3H), 6.86 (dd, J=6.9, 1.8 Hz, 2H), 5.96 (d, J=8.7 Hz, 1H), 4.55 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 139.5, 136.7, 128.9, 128.1, 128.0, 127.7, 127.1, 126.6, 120.2, 86.9, 53.9, 52.3; HRMS (ESI) calcd for C17H14N2NaO (M+Na)+ 285.0998, found 285.1006.
2-氨基-4-苯基-5-(对甲苯基)-4,5-二氢呋喃-3-甲腈 (3b): 白色固体, 产率68%. m.p. 131~137 ℃; 1H NMR (400 MHz, Chloroform-d) δ: 7.11-7.03 (m, 3H), 6.92~6.85 (m, 4H), 6.79 (d, J=8.2 Hz, 2H), 5.88 (d, J=8.8 Hz, 1H), 5.12 (s, 2H), 4.50 (d, J=8.8 Hz, 1H), 2.19 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ: 168.1, 137.6, 137.6, 132.2, 128.7, 128.5, 128.0, 127.1, 126.4, 119.1, 88.8, 57.0, 52.8, 21.1; HRMS (ESI) calcd for C18H16N2- NaO (M+Na)+ 299.1155, found 299.1165.
2-氨基-5-(4-甲氧基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3c): 白色固体, 产率64%. m.p. 127~130 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.36 (s, 2H), 7.04 (dd, J=18.5, 7.2 Hz, 3H), 6.86 (d, J=7.7 Hz, 4H), 6.62 (d, J=8.2 Hz, 2H), 5.89 (d, J=8.6 Hz, 1H), 4.47 (d, J=8.6 Hz, 1H), 3.59 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 158.7, 139.7, 129.0, 128.6, 128.2, 128.0, 127.1, 127.1, 113.3, 86.9, 55.3, 53.6, 52.3; HRMS (ESI) calcd for C18H16N2NaO2 (M+Na)+ 315.1104, found 315.1109.
2-氨基-5-(4-乙氧基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3d): 白色固体, 产率65%. m.p. 140~147 ℃; 1H NMR (400 MHz, Chloroform-d) δ: 7.08 (d, J=7.3 Hz, 3H), 6.87 (d, J=6.0 Hz, 2H), 6.80 (d, J=8.4 Hz, 2H), 6.59 (d, J=8.7 Hz, 2H), 5.87 (d, J=8.8 Hz, 1H), 5.01 (s, 2H), 4.48 (d, J=8.8 Hz, 1H), 3.89 (q, J=7.0 Hz, 2H), 1.33 (t, J=7.0 Hz, 3H); 13C NMR (100 MHz, Chloroform-d) δ: 167.9, 158.5, 137.5, 128.6, 128.0, 127.8, 127.1, 127.1, 118.9, 113.7, 88.8, 63.3, 56.9, 52.8, 14.7; HRMS (ESI) calcd for C19H18N2NaO2 (M+Na)+ 329.1260, found 329.1280.
2-氨基-5-(4-异丙氧基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3e): 白色固体, 产率43%. m.p. 119~126 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.36 (s, 2H), 7.04 (dd, J=17.4, 7.3 Hz, 2H), 6.88~6.78 (m, 4H), 6.62~6.56 (m, 2H), 5.88 (d, J=8.7 Hz, 1H), 4.45 (dd, J=10.7, 7.3 Hz, 2H), 1.12 (dd, J=6.0, 2.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 156.9, 139.7, 120.0, 128.4, 128.1, 128.0, 127.0, 120.3, 115.2, 87.0, 69.4, 53.5, 52.3, 22.1, 22.1; HRMS (ESI) calcd for C20H20N2NaO2 (M+Na)+ 343.1417, found 343.1428.
2-氨基-5-(4-异丙基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3f): 白色固体, 产率50%. m.p. 115~123 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.37 (s, 2H), 7.03 (d, J=7.2 Hz, 2H), 6.93 (d, J=7.9 Hz, 2H), 6.84 (dd, J=7.7, 4.5 Hz, 5H), 5.91 (d, J=8.6 Hz, 1H), 4.50 (d, J=8.6 Hz, 1H), 2.73~2.65 (m, 1H), 1.04 (d, J=6.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 147.8, 139.6, 134.1, 128.9, 128.1, 127.0, 126.6, 125.8, 120.3, 87.0, 53.8, 52.3, 33.4, 24.2, 24.2; HRMS (ESI) calcd for C20H20N2NaO (M+Na)+ 327.1468, found 327.1476.
2-氨基-5-(3-乙氧基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3g): 白色固体, 产率43%. m.p. 138~145 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.39 (s, 2H), 7.11~6.93 (m, 4H), 6.89~6.83 (m, 2H), 6.62~6.50 (m, 2H), 6.47 (d, J=2.2 Hz, 1H), 5.90 (d, J=8.7 Hz, 1H), 4.51 (d, J=8.7 Hz, 1H), 3.78 (dd, J=7.0, 3.9 Hz, 2H), 1.21 (d, J=7.1 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 158.2, 139.6, 138.2, 129.1, 128.9, 128.1, 127.1, 120.2, 118.8, 114.1, 112.7, 86.8, 63.3, 53.9, 52.2, 15.0; HRMS (ESI) calcd for C19H18N2NaO2 (M+Na)+ 329.1260, found 329.1276.
2-氨基-5-(4-(甲硫基)苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3h): 白色固体, 产率44%. m.p. 126~131 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.40 (s, 2H), 7.07 (d, J=7.3 Hz, 2H), 7.05~7.00 (m, 1H), 6.95 (d, J=8.2 Hz, 2H), 6.91~6.85 (m, 4H), 5.92 (d, J=8.6 Hz, 1H), 4.51 (d, J=8.7 Hz, 1H), 2.33 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 139.6, 137.5, 133.3, 128.9, 128.3, 127.2, 127.2, 125.3, 120.2, 86.7, 53.9, 52.2, 14.9; HRMS (ESI) calcd for C18H16N2NaOS (M+Na)+ 331.0876, found 331.0886.
2-氨基-5-([1'-联苯]-4-基)-4-苯基-4,5-二氢呋喃-3-腈(3i): 白色固体, 产率33%. m.p. 133~138 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.68 (dd, J=13.3, 7.8 Hz, 4H), 7.50~7.34 (m, 10H), 7.28 (dd, J=7.4, 5.8 Hz, 3H), 5.38 (d, J=6.4 Hz, 1H), 4.24 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 168.1, 142.4, 140.9, 140.0, 139.0, 129.5, 129.3, 129.0, 128.2, 127.9, 127.5, 127.2, 127.0, 119.9, 90.4, 56.0, 53.6; HRMS (ESI) calcd for C23H18N2NaO (M+Na)+ 361.1311, found 361.1319.
2-氨基-5-(4-氟苯基)-4-苯基-4,5-二氢呋喃-3-甲腈 (3j): 白色固体, 产率67%. m.p. 120~127 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.42 (s, 2H), 7.07 (t, J=7.2 Hz, 2H), 7.04~6.94 (m, 3H), 6.94~6.83 (m, 3H), 6.84 (t, J=1.4 Hz, 1H), 5.97 (d, J=8.7 Hz, 1H), 4.53 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 161.6 (d, J=243.5 Hz), 139.4, 133.0 (d, J=3.1 Hz), 128.9, 128.6 (d, J=8.3 Hz), 128.3, 127.2, 120.1, 114.8 (d, J=21.5 Hz), 86.2, 53.5, 52.2; 19F NMR (376 MHz, DMSO-d6) δ: -114.9; HRMS (ESI) calcd for C17H13FN2NaO (M+Na)+ 303.0904, found 303.0911.
2-氨基-5-(4-氯苯基)-4-苯基-4,5-二氢呋喃-3-甲腈 (3k): 白色固体, 产率70%. m.p. 119~126 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.47 (d, J=8.1 Hz, 2H), 7.44~7.32 (m, 6H), 7.24 (d, J=7.4 Hz, 3H), 5.35 (d, J=6.5 Hz, 1H), 4.17 (d, J=6.5 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 168.0, 142.1, 138.9, 133.6, 129.3, 129.2, 128.3, 127.9, 127.9, 119.8, 89.7, 56.0, 53.5; HRMS (ESI) calcd for C17H13ClN2NaO (M+Na)+ 319.0609, found 319.0616.
2-氨基-5-(4-溴苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3l): 白色固体, 产率55%. m.p. 123~130 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.61 (d, J=8.5 Hz, 2H), 7.42~7.33 (m, 4H), 7.31~7.21 (m, 5H), 5.34 (d, J=6.5 Hz, 1H), 4.15 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 168.0, 142.1, 139.3, 132.2, 129.3, 128.6, 127.9, 127.9, 122.2, 119.7, 89.7, 56.0, 53.5; HRMS (ESI) calcd for C17H13BrN2NaO (M+Na)+ 363.0103, found 363.0108.
2-氨基-5-(2-氯苯基)-4-苯基-4,5-二氢呋喃-3-甲腈 (3m): 白色固体, 产率66%. m.p. 128~134 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.46 (s, 1H), 7.23~7.17 (m, 1H), 7.07 (s, 3H), 7.12~7.01 (m, 3H), 6.99 (d, J=7.0 Hz, 1H), 6.96~6.89 (m, 2H), 6.12 (d, J=8.7 Hz, 1H), 4.65 (d, J=8.7 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 168.5, 139.3, 134.7, 130.8, 129.6, 129.1, 128.5, 128.2, 127.8, 127.4, 127.0, 119.9, 84.2, 54.2, 50.8; HRMS (ESI) calcd for C17H13ClN2NaO (M+Na)+ 319.0609, found 319.0617.
5-氨基-3-苯基-2,3-二氢-[2'-联呋喃]-4-甲腈(3n): 白色固体, 产率35%. m.p. 121~127 ℃; 1H NMR (400 MHz, Chloroform-d) δ: 7.51 (d, J=1.8 Hz, 1H), 7.35 (dd, J=8.0, 6.5 Hz, 2H), 7.27 (dt, J=6.8, 3.6 Hz, 3H), 6.46 (d, J=3.3 Hz, 1H), 6.44~6.39 (m, 1H), 5.31 (d, J=7.6 Hz, 1H), 4.93 (s, 2H), 4.64 (d, J=7.6 Hz, 1H); 13C NMR (100 MHz, Chloroform-d) δ: 166.7, 149.9, 144.1, 140.3, 129.0, 127.9, 127.4, 118.5, 110.7, 110.3, 85.1, 57.0, 52.0; HRMS (ESI) calcd for C15H12N2NaO2 (M+Na)+ 275.0791, found 275.0809.
2-氨基-5-(3,4-二甲基苯基)-4-苯基-4,5-二氢呋喃-3-甲腈(3o): 白色固体, 产率68%. m.p. 129~137 ℃; 1H NMR (400 MHz, Chloroform-d) δ: 7.36 (dd, J=8.0, 6.4 Hz, 2H), 7.31 (d, J=7.2 Hz, 1H), 7.25 (dd, J=6.8, 2.0 Hz, 2H), 7.15 (d, J=7.7 Hz, 1H), 7.08 (d, J=2.0 Hz, 1H), 7.04~6.97 (m, 1H), 5.25 (d, J=6.9 Hz, 1H), 5.10 (s, 2H), 4.31 (d, J=7.0 Hz, 1H), 2.28 (s, 6H); 13C NMR (100 MHz, Chloroform-d) δ: 167.3, 141.2, 137.6, 137.3, 136.4, 130.1, 129.0, 127.7, 127.5, 127.1, 123.5, 119.0, 92.7, 56.5, 56.4, 19.9, 19.6; HRMS (ESI) calcd for C19H18N2NaO (M+ Na)+ 313.1311, found 313.1331.
2-氨基-5-苯基-4-(对甲苯)-4,5-二氢呋喃-3-甲腈(3p): 白色固体, 产率64%. m.p. 116~123 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.38 (s, 2H), 7.07 (p, J=6.9 Hz, 3H), 6.96 (d, J=7.4 Hz, 2H), 6.85 (d, J=7.7 Hz, 2H), 6.74 (d, J=7.7 Hz, 2H), 5.93 (d, J=8.7 Hz, 1H), 4.48 (d, J=8.7 Hz, 1H), 2.10 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 168.8, 136.8, 136.5, 135.9, 128.8, 128.8, 128.0, 127.7, 126.5, 120.2, 86.9, 54.2, 51.9, 21.0; HRMS (ESI) calcd for C18H16N2NaO (M+Na)+ 299.1155, found 299.1160.
2-氨基-5-苯基-4-(对甲苯基)-4,5-二氢呋喃-3-甲腈 (3q): 白色固体, 产率67%. m.p. 119~125 ℃; 1H NMR (400 MHz, Chloroform-d) δ: 7.18~7.09 (m, 4H), 7.01 (t, J=1.9 Hz, 1H), 6.92 (td, J=7.5, 6.4, 3.1 Hz, 3H), 6.81~6.74 (m, 1H), 5.93 (d, J=8.9 Hz, 1H), 5.13 (s, 2H), 4.49 (d, J=8.9 Hz, 1H); 13C NMR (100 MHz, Chloroform-d) δ: 168.0, 140.1, 134.9, 131.6, 130.3, 129.5, 128.1, 128.0, 127.3, 126.1, 122.2, 118.5, 88.4, 56.7, 52.6; HRMS (ESI) calcd for C17H13BrN2NaO (M+Na)+ 363.0103, found 363.0107.