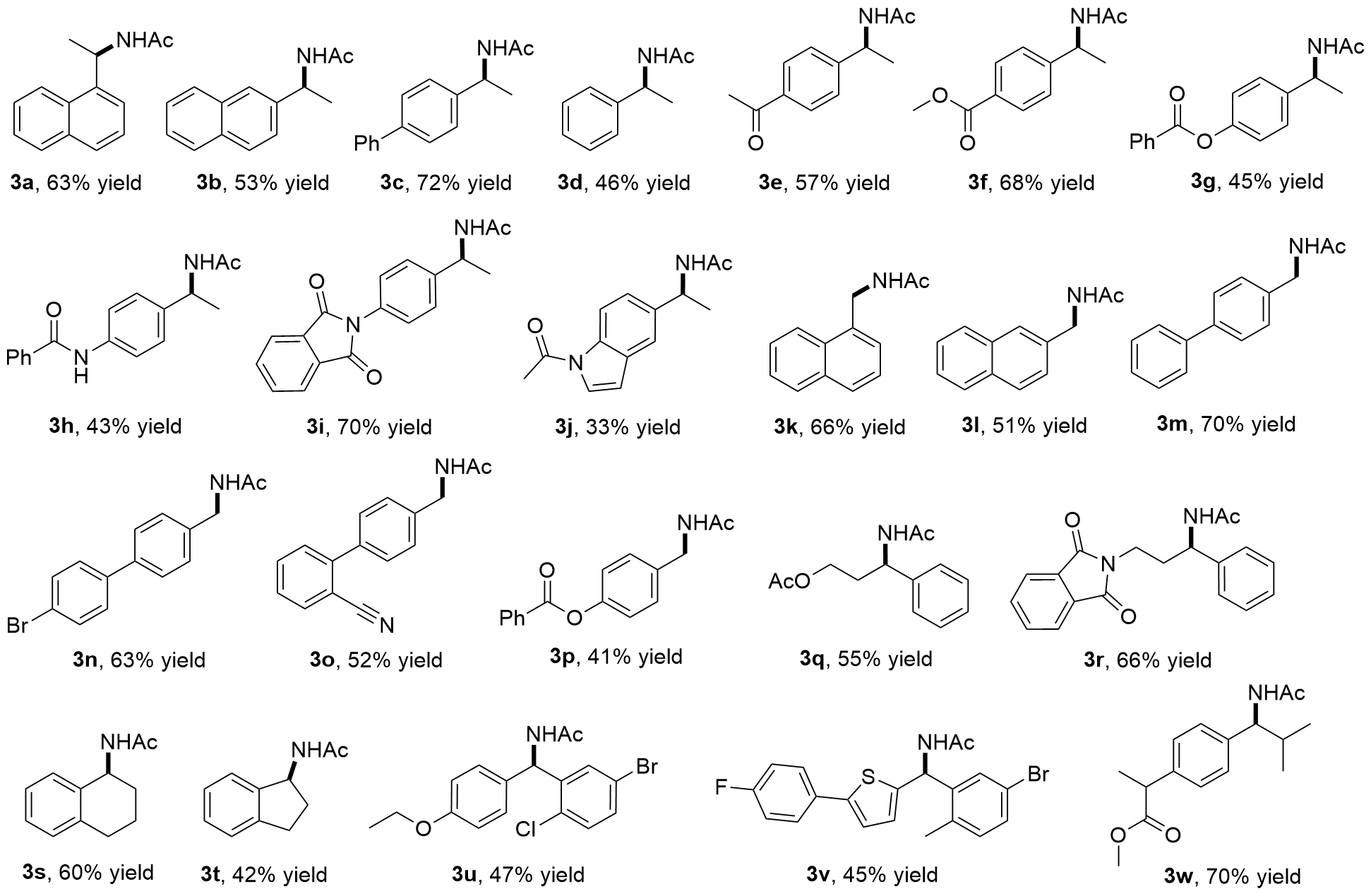
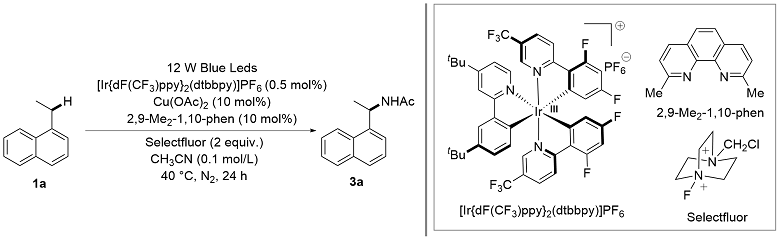
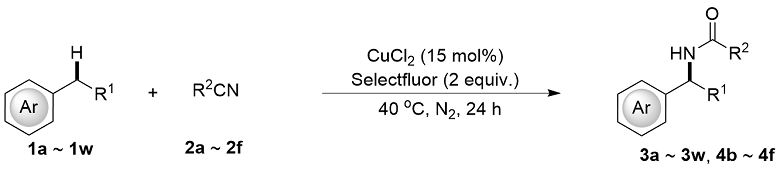
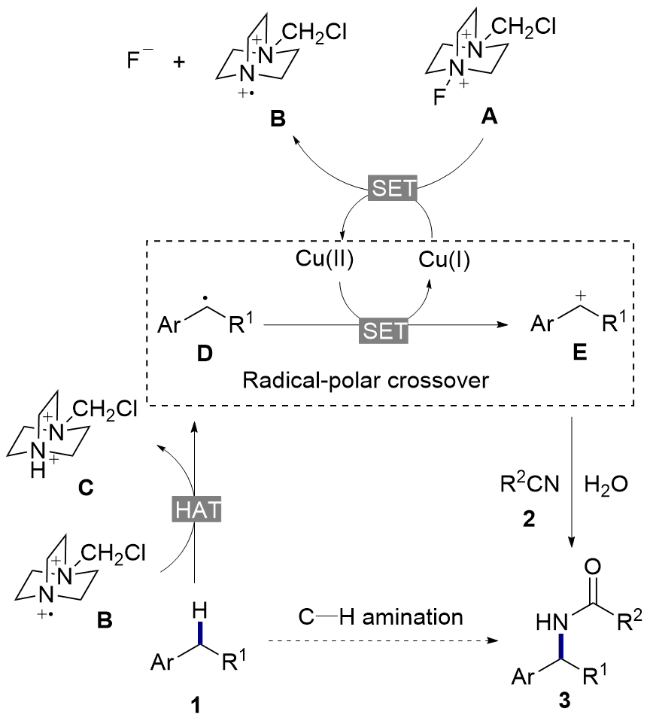
将苄基碳氢键化合物1(如果为固体, 0.5 mmol, 1.0 equiv.)、CuCl2 (0.15 mmol, 30 mol%)和Selectfluor (1.0 mmol, 2.0 equiv.)加到配备有特氟龙隔膜和磁力搅拌子的5 mL反应瓶中. 将反应瓶密封并置于N2气氛下, 通过隔膜注射CH3CN (2.5 mL, 0.2 mol/L)、苄基碳氢键化合物1(如果为液体, 0.5 mmol, 1.0 equiv.)入反应瓶中. 将密封的反应瓶置于40 ℃下反应24 h. 反应完成后, 用饱和NaHCO3水溶液稀释反应混合物, 乙酸乙酯(20 mL×3)萃取, 合并有机相, 用饱和食盐水(30 mL)洗涤, 无水硫酸钠干燥、过滤, 减压浓缩除去反应溶剂, 残余物经硅胶柱层析纯化[石油醚/乙酸乙酯作为洗脱剂]得到目标化合物3和4.
N-(1-(萘-1-基)乙基)乙酰胺(
3a)
[21]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 98~100 ℃. 67 mg, 产率63%.
1H NMR (400 MHz, CDCl
3)
δ: 8.10 (d,
J=8.1 Hz, 1H), 7.87 (d,
J=7.7 Hz, 1H), 7.80 (d,
J=7.9 Hz, 1H), 7.60~7.41 (m, 4H), 5.98~5.88 (m, 1H), 5.74 (br, 1H), 1.96 (s, 3H), 1.67 (d,
J=8.0 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 168.9, 138.2, 133.8, 131.1, 128.7, 128.3, 126.5, 125.8, 125.1, 123.4, 122.5, 44.6, 23.3, 20.6.
N-(1-(萘-2-基)乙基)乙酰胺(
3b)
[22]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 156~158 ℃. 56 mg, 产率53%.
1H NMR (400 MHz, CDCl
3)
δ: 7.82 (d,
J=7.5 Hz, 3H), 7.75 (s, 1H), 7.46 (dt,
J=14.3, 8.0 Hz, 3H), 5.85 (br, 1H), 5.33~5.24 (m, 1H), 2.01 (s, 3H), 1.58 (d,
J=6.7 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 169.1, 140.4, 133.3, 132.7, 128.5, 127.8, 127.6, 126.2, 125.9, 124.7, 124.5, 48.8, 23.5, 21.6.
N-(1-([1'-联苯]-4-基)乙基)乙酰胺(
3c)
[23]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 149~151 ℃. 86 mg, 产率72%.
1H NMR (400 MHz, DMSO-
d6)
δ: 8.33 (d,
J=7.6 Hz, 1H), 7.72~7.53 (m, 4H), 7.52~7.42 (m, 2H), 7.36 (dd,
J=17.2, 7.3 Hz, 3H), 5.00~4.89 (m, 1H), 1.85 (s, 3H), 1.36 (d,
J=6.8 Hz, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 168.2, 144.1, 140.0, 138.6, 128.9, 127.3, 126.6, 47.5, 22.7, 22.4.
N-(1-苯乙基)乙酰胺(
3d)
[24]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 74~76 ℃. 38 mg, 产率46%.
1H NMR (400 MHz, CDCl
3)
δ: 7.42~7.19 (m, 5H), 5.78 (br, 1H), 5.18~5.08 (m, 1H), 1.97 (s, 3H), 1.48 (d,
J=5.6 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 169.2, 143.2, 128.5, 127.2, 126.1, 48.7, 23.3, 21.7.
N-(1-(4-乙酰苯基)乙基)乙酰胺(
3e)
[24]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 89~91 ℃. 58 mg, 产率57%.
1H NMR (400 MHz, DMSO-
d6)
δ: 8.38 (d,
J=7.8 Hz, 1H), 7.90 (d,
J=8.3 Hz, 2H), 7.42 (d,
J=8.2 Hz, 2H), 4.98~4.88 (m, 1H), 2.55 (s, 3H), 1.84 (s, 3H), 1.33 (d,
J=7.1 Hz, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 197.5, 168.4, 150.4, 135.4, 129.2, 128.3, 126.2, 126.1, 47.8, 26.7, 22.6, 22.3.
4-(1-乙酰胺乙基)苯甲酸甲酯(
3f)
[23]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 94~96 ℃. 75 mg, 产率68%.
1H NMR (400 MHz, CDCl
3)
δ: 7.95 (d,
J=7.9 Hz, 2H), 7.33 (d,
J=7.8 Hz, 2H), 6.33 (d,
J=7.2 Hz, 1H), 5.17~5.04 (m, 1H), 3.87 (s, 3H), 1.95 (s, 3H), 1.43 (d,
J=6.9 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 169.4, 166.8, 148.6, 129.8, 128.9, 126.0, 52.0, 48.5, 23.2, 21.7.
4-(1-乙酰胺乙基)苯基苯甲酸酯(3g): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 184~187 ℃. 64 mg, 产率45%. 1H NMR (400 MHz, CDCl3) δ: 8.18 (d, J=7.1 Hz, 2H), 7.62 (d, J=7.4 Hz, 1H), 7.50 (t, J=7.5 Hz, 2H), 7.36 (d, J=8.3 Hz, 2H), 7.16 (d, J=8.4 Hz, 2H), 6.08 (d, J=6.3 Hz, 1H), 5.22~5.07 (m, 1H), 1.96 (s, 3H), 1.48 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 169.2, 165.2, 149.9, 140.8, 133.6, 130.1, 129.3, 128.5, 127.4, 121.8, 48.2, 23.3, 21.6. HRMS (ESI) calcd for C17H18NO3 [M+H]+ 284.1281, found 284.1287.
N-(4-(1-乙酰胺乙基)苯基)苯甲酰胺(3h): 洗脱剂: V(石油醚)/V(乙酸乙酯)=1/1, 白色固体, m.p. 220~223 ℃. 61 mg, 产率43%. 1H NMR (400 MHz, DMSO-d6) δ: 10.22 (br, 1H), 8.25 (d, J=7.9 Hz, 1H), 7.95 (d, J=7.3 Hz, 2H), 7.70 (d, J=8.2 Hz, 2H), 7.63~7.56 (m, 1H), 7.53 (t, J=7.4 Hz, 2H), 7.27 (d, J=8.2 Hz, 2H), 4.95~4.83 (m, 1H), 1.84 (s, 3H), 1.33 (d, J=6.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 168.1, 165.4, 140.0, 137.6, 134.9, 131.5, 128.4, 127.6, 126.2, 120.3, 47.3, 22.7, 22.4. HRMS (ESI) calcd for C17H19N2O2 [M+H]+ 283.1441, found 283.1444.
N-(1-(4-(1,3-二氧杂吲哚-2-基)苯基)乙基)乙酰胺(
3i)
[23]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=1/1, 白色固体, m.p. 234~236 ℃. 108 mg, 产率70%.
1H NMR (400 MHz, CDCl
3)
δ: 7.95 (dd,
J=5.1, 2.9 Hz, 2H), 7.79 (dd,
J=5.2, 2.9 Hz, 2H), 7.44 (dd,
J=21.1, 8.3 Hz, 4H), 5.79 (d,
J=6.9 Hz, 1H), 5.25~5.13 (m, 1H), 1.99 (s, 3H), 1.52 (d,
J=6.9 Hz, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 168.3, 167.1, 144.9, 134.7, 131.5, 130.2, 127.2, 126.4, 123.4, 47.6, 22.6, 22.4.
N-(1-(1-乙酰基-1H-吲哚-5-基)乙基)乙酰胺(3j): 洗脱剂: V(石油醚)/V(乙酸乙酯)=1/1, 白色固体, m.p. 201~204 ℃. 40 mg, 产率33%. 1H NMR (400 MHz, DMSO-d6) δ: 8.39 (d, J=7.8 Hz, 1H), 8.27 (d, J=8.2 Hz, 1H), 8.17 (d, J=8.0 Hz, 2H), 8.09 (s, 1H), 7.47 (ddd, J=20.9, 15.3, 7.4 Hz, 3H), 5.14~5.02 (m, 1H), 2.87 (s, 3H), 1.87 (s, 3H), 1.44 (d, J=6.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 170.4, 168.3, 140.4, 138.4, 136.9, 127.4, 125.6, 123.6, 120.0, 117.4, 116.3, 116.1, 47.7, 27.5, 22.7. HRMS (ESI) calcd for C14H17N2O2 [M+H]+ 245.1285, found 245.1290.
N-(萘-1-基甲基)乙酰胺(3k): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 127~129 ℃. 66 mg, 产率66%. 1H NMR (400 MHz, DMSO-d6) δ: 8.39 (br, 1H), 8.07 (d, J=7.6 Hz, 1H), 7.95 (d, J=7.9 Hz, 1H), 7.85 (d, J=7.2 Hz, 1H), 7.64~7.52 (m, 2H), 7.50~7.35 (m, 2H), 4.72 (d, J=5.5 Hz, 2H), 1.90 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 134.7, 133.3, 130.9, 128.5, 127.6, 126.2, 125.8, 125.6, 125.4, 123.5, 40.2, 22.6. HRMS (ESI) calcd for C13H14NO [M+H]+ 200.1070, found 200.1077.
N-(萘-2-基甲基)乙酰胺(
3l)
[24]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 111~113 ℃. 51 mg, 产率51%.
1H NMR (400 MHz, DMSO-
d6)
δ: 8.45 (br, 1H), 7.87 (d,
J=7.9 Hz, 3H), 7.74 (s, 1H), 7.54~7.45 (m, 2H), 7.42 (d,
J=8.5 Hz, 1H), 4.42 (d,
J=5.7 Hz, 2H), 1.91 (s, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 169.2, 137.2, 132.9, 132.0, 127.9, 127.5, 126.1, 125.9, 125.6, 125.3, 42.3, 22.6.
N-([1'-联苯]-4-基甲基)乙酰胺(
3m)
[23]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 162~164 ℃. 79 mg, 产率70%.
1H NMR (400 MHz, DMSO-
d6)
δ: 8.38 (br, 1H), 7.63 (dd,
J=11.0, 8.3 Hz, 4H), 7.46 (t,
J=7.6 Hz, 2H), 7.35 (dd,
J=11.4, 4.6 Hz, 3H), 4.29 (d,
J=5.8 Hz, 2H), 1.89 (s, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 169.1, 140.0, 138.9, 138.7, 128.9, 127.9, 127.3, 126.6, 126.6, 41.8, 22.6.
N-((4'-溴-[1'-联苯]-4-基)甲基)乙酰胺(3n): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 171~173 ℃. 96 mg, 产率63%. 1H NMR (400 MHz, DMSO-d6) δ: 8.39 (br, 1H), 7.63 (t, J=7.1 Hz, 5H), 7.34 (d, J=7.8 Hz, 2H), 4.28 (d, J=5.7 Hz, 2H), 1.89 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 169.1, 139.4, 139.1, 137.3, 131.8, 128.6, 128.0, 126.5, 120.8, 41.8, 22.6. HRMS (ESI) calcd for C15H15BrNO [M+H]+ 304.0332, found 304.0337.
N-((2'-氰基-[1'-联苯]-4-基)甲基)乙酰胺(
3o)
[14]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 129~131 ℃. 65 mg, 产率52%.
1H NMR (400 MHz, CDCl
3)
δ: 7.75 (d,
J=7.7 Hz, 1H), 7.64 (t,
J=7.5 Hz, 1H), 7.50 (d,
J=8.3 Hz, 2H), 7.44 (dd,
J=14.1, 6.2 Hz, 2H), 7.38 (d,
J=7.8 Hz, 2H), 6.18 (br, 1H), 4.46 (d,
J=5.4 Hz, 2H), 2.02 (s, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 170.2, 145.0, 138.9, 137.2, 133.7, 132.9, 129.9, 129.0, 128.1, 127.6, 118.7, 111.1, 43.3, 23.2.
4-(乙酰胺甲基)苯基苯甲酸酯(
3p)
[11]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 177~178 ℃. 55 mg, 产率41%.
1H NMR (400 MHz, CDCl
3)
δ: 8.20 (d,
J=7.1 Hz, 2H), 7.65 (t,
J=7.4 Hz, 1H), 7.52 (t,
J=7.7 Hz, 2H), 7.36 (d,
J=8.4 Hz, 2H), 7.19 (d,
J=8.5 Hz, 2H), 5.73 (br, 1H), 4.46 (d,
J=5.7 Hz, 2H), 2.05 (s, 3H);
13C NMR (100 MHz, DMSO-
d6)
δ: 169.2, 164.7, 149.4, 137.4, 134.0, 129.8, 129.0, 128.4, 121.7, 41.6, 22.6.
3-乙酰胺-3-苯丙基乙酸酯(
3q)
[25]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 89~91 ℃. 65 mg, 产率55%.
1H NMR (400 MHz, CDCl
3)
δ: 7.40~7.20 (m, 5H), 5.93 (br, 1H), 5.13 (dd,
J=14.2, 6.8 Hz, 1H), 4.14~3.98 (m, 2H), 2.24~2.10 (m, 2H), 2.00 (d,
J=10.0 Hz, 6H);
13C NMR (100 MHz, CDCl
3)
δ: 171.0, 169.3, 141.1, 128.8, 127.7, 126.5, 61.4, 50.7, 34.7, 23.4, 20.9.
N-(3-(1,3-二氧杂吲哚-2-基)-1-苯基丙基)乙酰胺(3r): 洗脱剂: V(石油醚)/V(乙酸乙酯)=1/1, 白色固体, m.p. 191~195 ℃. 106 mg, 产率66%. 1H NMR (400 MHz, CDCl3) δ: 7.72 (d, J=30.8 Hz, 4H), 7.28~7.16 (m, 4H), 7.11 (t, J=6.9 Hz, 1H), 6.17 (d, J=7.8 Hz, 1H), 5.17~5.08 (m, 1H), 3.73 (dt, J=14.0, 7.5 Hz, 2H), 2.25 (d, J=6.6 Hz, 2H), 2.03 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 169.5, 168.2, 140.9, 133.8, 131.9, 128.6, 127.3, 126.3, 123.1, 50.9, 35.0, 33.8, 23.4. HRMS (ESI) calcd for C19H19N2O3 [M+H]+ 323.1390, found 323.1399.
N-(1,2,3,4-四氢萘-1-基)乙酰胺(
3s)
[21]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 146~148 ℃. 57 mg, 产率60%.
1H NMR (400 MHz, CDCl
3)
δ: 7.33~7.24 (m, 1H), 7.16 (dd,
J=8.7, 5.0 Hz, 2H), 7.14~7.05 (m, 1H), 5.78 (br, 1H), 5.24~5.12 (m, 1H), 2.92~2.68 (m, 2H), 2.01 (s, 3H), 1.81 (d,
J=11.6 Hz, 4H);
13C NMR (100 MHz, CDCl
3)
δ: 169.2, 137.6, 136.6, 129.1, 128.7, 127.2, 126.2, 47.4, 30.1, 29.2, 23.5, 19.9.
N-(2,3-二氢-1
H-茚-1-基)乙酰胺(
3t)
[21]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 152~154 ℃. 37 mg, 产率42%.
1H NMR (400 MHz, CDCl
3)
δ: 7.34~7.17 (m, 4H), 5.72 (br, 1H), 5.47 (q,
J=7.6 Hz, 1H), 3.02~2.93 (m, 1H), 2.91~2.81 (m, 1H), 2.63~2.52 (m, 1H), 2.02 (s, 3H), 1.87~1.74 (m, 1H);
13C NMR (100 MHz, CDCl
3)
δ: 169.8, 143.4, 143.1, 127.9, 126.7, 124.8, 124.0, 54.7, 34.0, 30.2, 23.4.
N-((5-溴-2-氯苯基)(4-乙氧基苯基)甲基)乙酰胺(3u): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 179~182 ℃. 90 mg, 产率47%. 1H NMR (400 MHz, CDCl3) δ: 7.50 (s, 1H), 7.33 (d, J=8.3 Hz, 1H), 7.19 (d, J=8.1 Hz, 1H), 7.05 (d, J=7.8 Hz, 2H), 6.80 (d, J=7.7 Hz, 2H), 6.66 (d, J=7.0 Hz, 1H), 6.32 (d, J=7.1 Hz, 1H), 3.97 (q, J=6.6 Hz, 2H), 1.95 (s, 3H), 1.38 (t, J=6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 169.3, 158.4, 141.2, 132.3, 131.5, 131.4, 131.0, 130.8, 128.7, 120.6, 114.5, 63.3, 54.1, 22.9, 14.7. HRMS (ESI) calcd for C17H18BrClNO2 [M+H]+ 382.0204, found 382.0211.
N-((5-溴-2-甲基苯基)(5-(4-氟苯基)噻吩-2-基)甲基)乙酰胺(3v): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 183~186 ℃. 94 mg, 产率45%. 1H NMR (400 MHz, CDCl3) δ: 7.54~7.46 (m, 2H), 7.44 (s, 1H), 7.35 (d, J=7.9 Hz, 1H), 7.13~6.94 (m, 4H), 6.67 (s, 1H), 6.50 (d, J=7.5 Hz, 1H), 6.20 (d, J=7.2 Hz, 1H), 2.30 (s, 3H), 2.08 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 169.0, 163.6, 161.1, 143.5, 143.4, 141.3, 135.0, 132.4, 130.8, 130.2, 128.7, 127.3, 127.0, 122.7, 119.9, 115.9, 115.7, 49.7, 23.1, 18.8. HRMS (ESI) calcd for C20H18BrFNOS [M+H]+ 418.0271, found 418.0277.
2-(4-(1-乙酰胺-2-甲基丙基)苯基)丙酸甲酯(
3w)
[13]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 105~107 ℃. 97 mg, 产率70%.
1H NMR (400 MHz, CDCl
3)
δ: 7.30~7.14 (m, 4H), 6.05 (d,
J=8.0 Hz, 1H), 4.73 (t,
J=8.2 Hz, 1H), 3.75~3.69 (m, 1H), 3.65 (s, 3H), 2.08~2.00 (m, 1H), 1.98 (s, 3H), 1.48 (d,
J=6.6 Hz, 3H), 0.96 (d,
J=5.9 Hz, 3H), 0.82 (d,
J=6.0 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 175.0, 169.4, 140.5, 139.2, 127.5, 127.2, 58.8, 52.0, 44.9, 33.2, 23.4, 19.7, 18.8, 18.50.
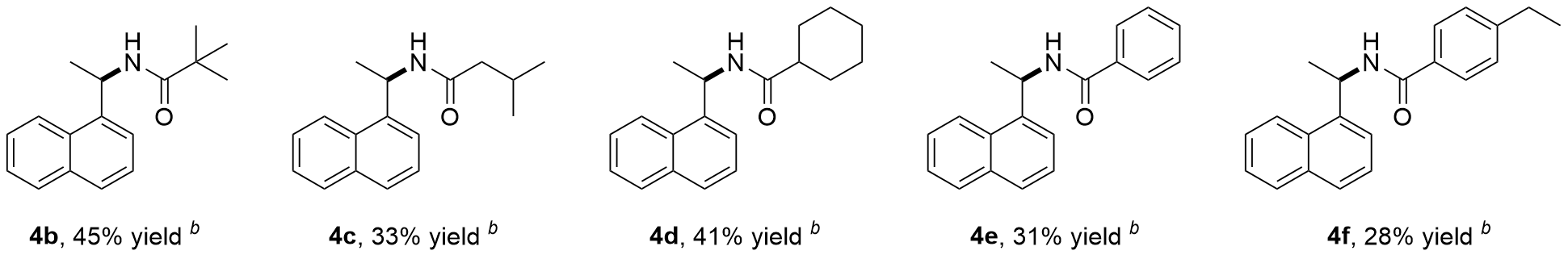
N-(1-(萘-1-基)乙基)新戊酰胺(
4b)
[26]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 143~145 ℃. 57 mg, 产率45%.
1H NMR (400 MHz, CDCl
3)
δ: 8.05 (d,
J=8.0 Hz, 1H), 7.86 (d,
J=7.5 Hz, 1H), 7.80 (d,
J=8.0 Hz, 1H), 7.57~7.39 (m, 4H), 5.94~5.86 (m, 1H), 5.84 (s, 1H), 1.66 (d,
J=6.4 Hz, 3H), 1.17 (s, 9H);
13C NMR (100 MHz, CDCl
3)
δ: 177.2, 138.5, 131.2, 128.7, 128.3, 126.4, 125.8, 125.1, 123.5, 122.4, 44.5, 38.6, 27.5, 20.5.
3-甲基-N-(1-(萘-1-基)乙基)丁酰胺(4c): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 151~153 ℃. 42 mg, 产率33%. 1H NMR (400 MHz, CDCl3) δ: 8.10 (d, J=8.0 Hz, 1H), 7.86 (d, J=7.4 Hz, 1H), 7.79 (d, J=8.0 Hz, 1H), 7.56~7.41 (m, 4H), 6.01~5.88 (m, 1H), 5.77 (d, J=6.7 Hz, 1H), 2.18~2.06 (m, 1H), 2.00 (d, J=6.7 Hz, 2H), 1.66 (d, J=6.6 Hz, 3H), 0.92 (dd, J=15.3, 6.5 Hz, 7H); 13C NMR (100 MHz, CDCl3) δ: 171.3, 138.2, 133.9, 131.1, 128.7, 128.3, 126.5, 125.8, 125.1, 123.5, 122.5, 46.1, 44.3, 26.1, 22.42, 22.36, 20.5. HRMS (ESI) calcd for C17H22NO [M+H]+ 256.1696, found 256.1701.
N-(1-(萘-1-基)乙基)环己甲酰胺(
4d)
[26]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 159~161 ℃. 58 mg, 产率41%.
1H NMR (400 MHz, CDCl
3)
δ: 8.06 (d,
J=8.2 Hz, 1H), 7.86 (d,
J=7.5 Hz, 1H), 7.79 (d,
J=8.0 Hz, 1H), 7.56~7.47 (m, 3H), 7.44 (t,
J=7.6 Hz, 1H), 5.96~5.86 (m, 1H), 5.75 (d,
J=7.5 Hz, 1H), 2.08~1.98 (m, 1H), 1.89~1.69 (m, 5H), 1.65 (d,
J=6.7 Hz, 4H), 1.51~1.36 (m, 2H), 1.27~1.13 (m, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 174.9, 138.4, 133.9, 131.2, 128.7, 128.3, 126.4, 125.8, 125.1, 123.5, 122.4, 45.6, 44.2, 29.7, 29.5, 25.64, 25.61, 20.5.
N-(1-(萘-1-基)乙基)苯甲酰胺(
4e)
[27]: 洗脱剂:
V(石油醚)/
V(乙酸乙酯)=2/1, 白色固体, m.p. 183~185 ℃. 43 mg, 产率31%.
1H NMR (400 MHz, CDCl
3)
δ: 8.17 (d,
J=8.2 Hz, 1H), 7.88 (d,
J=7.7 Hz, 1H), 7.82 (d,
J=8.2 Hz, 1H), 7.73 (d,
J=7.9 Hz, 2H), 7.60 (d,
J=7.1 Hz, 1H), 7.56~7.42 (m, 4H), 7.37 (t,
J=7.4 Hz, 2H), 6.46 (d,
J=7.1 Hz, 1H), 6.18~6.07 (m, 1H), 1.78 (d,
J=6.9 Hz, 3H);
13C NMR (100 MHz, CDCl
3)
δ: 166.4, 138.1, 134.4, 133.9, 131.4, 131.2, 128.7, 128.5, 126.9, 126.6, 125.9, 125.2, 123.4, 122.6, 45.2, 20.6.
4-乙基-N-(1-(萘-1-基)乙基)苯甲酰胺(4f): 洗脱剂: V(石油醚)/V(乙酸乙酯)=2/1, 白色固体, m.p. 187~189 ℃. 42 mg, 产率28%. 1H NMR (400 MHz, CDCl3) δ: 8.17 (d, J=8.3 Hz, 1H), 7.88 (d, J=7.5 Hz, 1H), 7.82 (d, J=8.2 Hz, 1H), 7.66 (d, J=8.2 Hz, 2H), 7.60 (d, J=7.1 Hz, 1H), 7.56~7.43 (m, 3H), 7.21 (d, J=8.1 Hz, 2H), 6.31 (d, J=7.6 Hz, 1H), 6.17~6.08 (m, 1H), 2.66 (q, J=7.6 Hz, 2H), 1.78 (d, J=6.7 Hz, 3H), 1.21 (t, J=7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 166.4, 148.1, 138.2, 133.9, 131.7, 131.2, 128.7, 128.4, 128.0, 127.0, 126.6, 125.8, 125.2, 123.5, 122.6, 45.1, 28.7, 20.6, 15.3. HRMS (ESI) calcd for C21H22NO [M+H]+ 304.1696, found 304.1699.