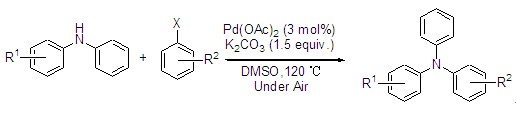
| [1] Liu, T.; Sun, J.; Li, R.; Tao, X.-C. Chin. J. Org. Chem. 2011, 31, 1799. (刘涛平, 孙杰, 李瑞, 陶晓春, 有机化学, 2011, 31, 1799.)[2] Chan, H. S. O.; Ng, S. C.; Leong, L. S.; Tan, K. L. Synth. Met. 1995, 68, 199. [3] Fang, Q.; Xua, B.; Jiang, B.; Fu, H. T.; Zhu, W. Q.; Jiang, X. Y.; Zhang, Z. L. Synth. Met. 2005, 105, 206. [4] Zhang, L.; Li, B.; Lei, B.; Hong, Z.; Li, W. J. Lumin. 2008, 128, 67. [5] Sengupta, S. Tetrahedron Lett. 2003, 44, 307. [6] Zheng, B.; Niu, H.-J.; Bai, X.-Z. Prog. Chem. 2008, 20, 828. (郑冰, 牛海军, 白续铎, 化学进展, 2008, 20, 828.)[7] Ramu, B.; Swamy, G. N. J. Chem. Pharm. Sci. 2009, 2, 73. [8] Wu, X.; Davis, A. P.; Lambert, P. C.; Kraig Steffen, L.; Toy, O.; Fry, A. J. Tetrahedron 2009, 65, 2408. [9] Liu, J. CN 101012171A, 2007. (刘坚, 中国发明专利, CN101012171A, 2007.)[10] Tao, X.-C.; Yang, X.-H.; Huang, Y.-Z.; Sun, J.; Cai, L.-Z.; Cao, X.-J.; Zhu, Z. CN101538210A, 2009. (陶晓春, 杨兴淮, 黄应钊, 孙杰, 蔡良珍, 曹雄杰, 朱哲, 中国发明专利, CN101538210A, 2009.)[11] Liu, P.; Li, S.-H.; Li, L.-M.; Wang, X.-Y.; Yin, Y.-Q.; Zhang, Y.-H. Prog. Chem. 2005, 17, 286. (刘蒲, 李三华, 李利民, 王向宇, 殷元琪, 张玉华, 化学进展, 2005, 17, 286.)[12] Hartwig, J. F. Angew. Chem. Int. Ed. Engl. 1998, 37, 2046.[13] Beilfeid, A. J.; Brown, G. R.; Fobiser, A. J. Tetrahadron 1999, 55, 11399. [14] Yang, B. H.; Buchwald, S. L. J. Organomet. Chem. 1999, 576, 125.[15] Zhang, Z.-F.; Zhou, W.-C. Chin. J. Org. Chem. 2002, 22, 685. (张贞发, 周伟澄, 有机化学, 2002, 22, 685.)[16] Guram, A. S.; Rennels, R. A.; Buchwald, S. L. Angew. Chem., Int. Ed. Engl. 1995, 34, 1348. [17] Fors, B. P.; Dooleweerdt, K.; Zeng, Q.; Buchwald, S. L. Tetrahedron 2009, 65, 6576. [18] Stambuli, J. P.; Buehl, M.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 9346. [19] Urgaonkar, S.; Nagarajian, M.; Verkand, J. G. Org. Lett. 2003, 5, 815. [20] Hartwig, J. F.; Kawatsura, M.; Hauck, S. I. J. Am. Chem. Soc. 1999, 64, 5575. [21] Han, W.; Liu, C.; Jin, Z.-L. Adv. Synth. Catal. 2008, 350, 501. [22] Liu, C.; Yang, W.-B. Chem. Commun. 2009, 6267. [23] Wu, N.; Li, X.; Xu, X.; Wang, Y.; Xu, Y.; Chen, X. Lett. Org. Chem. 2010, 7, 11. [24] Liu, C.; Ni, Q.-J.; Qiu, J.-S. Eur. J. Org. Chem. 2011, (16), 3009. [25] Tan, J.; Tang, W.; Sun, Y.; Jiang, Z.; Chen, F.; Xu, L.; Fan, Q.; Xiao, J. Tetrahedron 2011, 67, 6206. [26] Li, H.; Yang, M.; Qi, Y.; Xue, J. Eur. J. Org. Chem. 2011, 14, 2662. [27] Gholap, A. R.; Venkatesan, K.; Pasricha, R.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V. J. Org. Chem. 2005, 70, 4869. [28] Zeng, Q.; Liu, H.; Cui, X.; Mi, A.; Jiang, Y.; Li, X.; Choi, M. C. K.; Chan, A. S. C. Tetrahedron: Asymmetry 2002, 13, 115. [29] Zeng, Q.; Wang, H.; Wang, T.; Cai, Y.; Weng, W.; Zhao, Y. Adv. Synth. Catal. 2005, 347, 1933. [30] Zeng, Q.-L.; Tang, H.-Y.; Zhang, S.; Liu, J.-C. Chin. J. Chem. 2008, 26, 1435. [31] Zeng, Q.; Gao, Y.; Dong, J.; Weng, W.; Zhao, Y. Tetrahedron: Asymmetry 2011, 22, 717. |