化学学报 ›› 2019, Vol. 77 ›› Issue (11): 1115-1128.DOI: 10.6023/A19070265 上一篇 下一篇
综述
李钊abc, 王忠abc*( ), 班丽卿abc, 王建涛abc, 卢世刚abc
), 班丽卿abc, 王建涛abc, 卢世刚abc
投稿日期:2019-07-16
发布日期:2019-10-09
通讯作者:
王忠
E-mail:wzwz99@126.com
作者简介:李钊, 男, 1992年生, 硕士生. 2014年毕业于西北师范大学, 获得环境工程学士学位. 2014~2017年, 先后在锂电企业和中科院电工研究所从事锂离子电池材料和器件的研发工作. 2017年进入北京有色金属研究总院攻读材料科学与工程硕士学位.主要进行高性能富锂锰基正极材料的结构和界面研究|王忠, 男, 1967年生, 教授, 博士生导师. 2007年在北京科技大学获博士学位, 同年进入北京有色金属研究总院工作至今, 主要从事锂离子电池材料的结构和电化学性能的研究|卢世刚, 男, 1966年生, 教授, 博士生导师. 1993年在莫斯科大学获得化学博士学位.现任北京有色金属研究总院副总工程师, 国家动力电池创新中心首席专家, 承担新一代动力电池及材料的国家重点研发项目
基金资助:
Li Zhaoabc, Wang Zhongabc*( ), Ban Liqinabc, Wang Jiantaoabc, Lu Shigangabc
), Ban Liqinabc, Wang Jiantaoabc, Lu Shigangabc
Received:2019-07-16
Published:2019-10-09
Contact:
Wang Zhong
E-mail:wzwz99@126.com
Supported by:文章分享
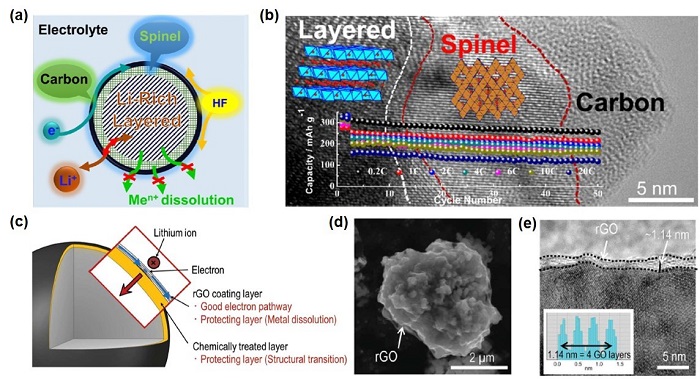
随着电动汽车和储能电站等电力设备的快速发展,对高能量密度的锂离子电池的需求日益增加.高比容量(>250 mAh·g-1)的富锂锰基正极材料,有望成为锂离子电池实现高比能量(>350 Wh·kg-1)的关键正极材料.富锂锰基正极材料的Li2MnO3相和晶格氧参与电化学反应使其拥有了高容量,但这也导致表面结构和成分容易发生变化,进而造成富锂锰基正极材料存在着诸如首次库伦效率低、倍率性能差和循环后电压和容量衰减严重等问题.因此,本文综述了富锂锰基正极材料的表面包覆、表面掺杂和表面化学处理三种表面改性方法,并进一步讨论了三种表面改性方法对材料性能提升的机制机理和优缺点.在此基础上,介绍了近些年基于多方法的表面联合改性工作.通过对富锂锰基正极材料进行表面联合改性,不仅可以改善其结构稳定性和抑制电极/电解液界面副反应,而且可以缓解其在循环过程中不断发生的结构转变和晶格氧的析出问题.最后,对富锂锰基正极材料表面改性研究方向进行了总结和展望.
李钊, 王忠, 班丽卿, 王建涛, 卢世刚. 富锂锰基正极材料的表面改性研究进展[J]. 化学学报, 2019, 77(11): 1115-1128.
Li Zhao, Wang Zhong, Ban Liqin, Wang Jiantao, Lu Shigang. Recent Advances on Surface Modification of Li- and Mn-Rich Cathode Materials[J]. Acta Chimica Sinica, 2019, 77(11): 1115-1128.



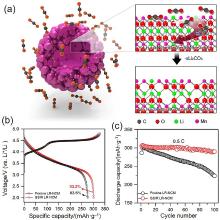

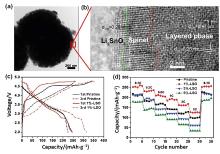
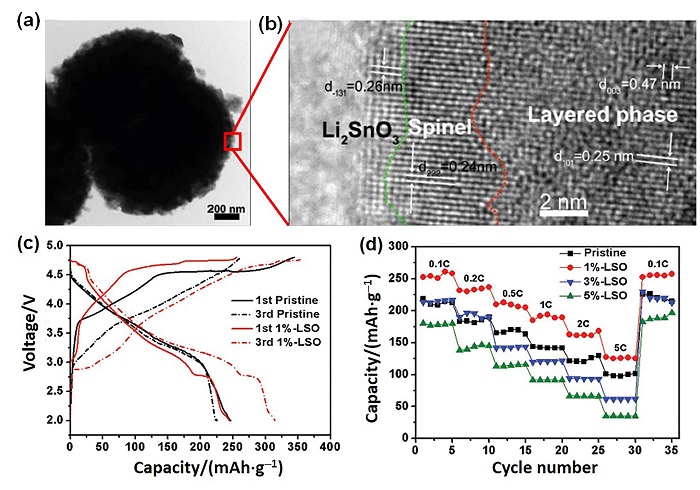
| 表面改性方法 | 优势 | 不足 | 电化学性能 | 参考文献 | ||
| 表面包覆 | 惰性包覆层 | 抑制电极/电解液界面副反应, 提升电极材料的循环和热稳定性. | 过量的惰性包覆物会阻碍电极的电荷转移, 并降低电极有效活性质量. | Al2O3包覆层, 0.1 C, 50 ℃下首放310 mAh?g-1, 循环30次容量没有衰减. | [ | |
| AlF3包覆层, C/3室温下循环150次, 容量没有衰减. C/3, 60 ℃下循环150次容量保持率在93.6%. | [ | |||||
| 活性包覆层 | 提升材料的首次放电比容量和首次库伦效率. | 在反应中也会与电解液发生反应, 消耗部分Li+, 导致材料循环保持率会有所降低. | MnO2包覆层, 0.1 C下, 首放299 mAh? g-1, 首库88%. | [ | ||
| 离子电导 包覆层 | 能够为电极材料提供活性Li+, 提高材料的长循环性能. | 混合离子导体对材料表面的均匀包覆还存在一定难度. | 5 wt% LiFePO4包覆层, 0.1 C下首放282.8 mAh?g-1, 循环120次后容量保持率98.1%. | [ | ||
| 电子电导 包覆层 | 提升材料表面的电子电导能力, 提高材料的倍率性能. | 碳材料、石墨烯和导电聚合物与材料的均匀分散难度大, 且石墨烯和导电聚合物成本高. | 多巴胺作为碳源, 10 C下的放电容量可达200 mAh?g-1, 循环50次后, 容量保持在176 mAh?g-1. | [ | ||
| 混合包覆层 | 利用两种及以上不同特性包覆层的协同优势, 去同时提高材料的倍率性能和循环稳定性. | 比单一包覆层的用量多, 合成步骤也多, 包覆物的选择和实用的包覆方法有限. | AlPO4和石墨烯复合包覆层, 5 C放电容量为105 mAh?g-1, 在55 ℃高温0.1 C下循环45次, 容量保持率为90.1%. | [ | ||
| 表面掺杂 | 增强材料表面结构稳定性, 抑制材料表面发生的相转变和晶格氧流失, 缓解电压衰减问题. | 可选择的掺杂离子种类较少, 离子在表层的扩散深度不容易控制.直接对成品进行表面掺杂, 容易增大材料表面的晶格缺陷. | Na+掺杂进入Li层, 在层间起到支柱的作用. 25 mA?g-1下, 材料首放和首库提升至286 mAh?g-1和87%. | [ | ||
| Ru4+取代了材料表面的部分Mn4+, 在0.1 C下, 首放280 mAh?g-1. 0.1 C下循环100次后, 容量保持率为98.1%. | [ | |||||
| 表面化学处理 | 活化材料表面Li2MnO3相, 减少材料的首次不可逆容量, 提高材料的首次库伦效率. | 处理后对材料表面结构会造成破坏, 会降低材料的循环稳定性.用气体处理材料比液体处理的反应温和, 对材料表面结构影响也较小. | (NH4)2SO4溶液处理材料, 300 mA?g-1下首放可达270 mAh?g-1, 首库从原来的76%提升至95%. | [ | ||
| CO2与材料发生气固界面反应, 在材料表面形成了均匀的一层氧空位.首放301 mAh?g-1, 首库高达93.8%. | [ | |||||
| 表面联合改性 | 避免了单一表面改性方式的不足, 将会成为富锂锰基正极材料的表面改性研究的一个趋势. | 涉及的工艺步骤较多, 且对两种以上表面改性方法的协同改性机理的认识还不足, 需要进一步去研究优化和简化现有方法. | Li2SnO3包覆和表面Sn4+掺杂材料. 1 wt%包覆量, 在5 C下放电比容量126 mAh?g-1, 1 C, 55 ℃下循环70次, 放电比容量保持在235.1 mAh?g-1. | [ | ||
| 表面改性方法 | 优势 | 不足 | 电化学性能 | 参考文献 | ||
| 表面包覆 | 惰性包覆层 | 抑制电极/电解液界面副反应, 提升电极材料的循环和热稳定性. | 过量的惰性包覆物会阻碍电极的电荷转移, 并降低电极有效活性质量. | Al2O3包覆层, 0.1 C, 50 ℃下首放310 mAh?g-1, 循环30次容量没有衰减. | [ | |
| AlF3包覆层, C/3室温下循环150次, 容量没有衰减. C/3, 60 ℃下循环150次容量保持率在93.6%. | [ | |||||
| 活性包覆层 | 提升材料的首次放电比容量和首次库伦效率. | 在反应中也会与电解液发生反应, 消耗部分Li+, 导致材料循环保持率会有所降低. | MnO2包覆层, 0.1 C下, 首放299 mAh? g-1, 首库88%. | [ | ||
| 离子电导 包覆层 | 能够为电极材料提供活性Li+, 提高材料的长循环性能. | 混合离子导体对材料表面的均匀包覆还存在一定难度. | 5 wt% LiFePO4包覆层, 0.1 C下首放282.8 mAh?g-1, 循环120次后容量保持率98.1%. | [ | ||
| 电子电导 包覆层 | 提升材料表面的电子电导能力, 提高材料的倍率性能. | 碳材料、石墨烯和导电聚合物与材料的均匀分散难度大, 且石墨烯和导电聚合物成本高. | 多巴胺作为碳源, 10 C下的放电容量可达200 mAh?g-1, 循环50次后, 容量保持在176 mAh?g-1. | [ | ||
| 混合包覆层 | 利用两种及以上不同特性包覆层的协同优势, 去同时提高材料的倍率性能和循环稳定性. | 比单一包覆层的用量多, 合成步骤也多, 包覆物的选择和实用的包覆方法有限. | AlPO4和石墨烯复合包覆层, 5 C放电容量为105 mAh?g-1, 在55 ℃高温0.1 C下循环45次, 容量保持率为90.1%. | [ | ||
| 表面掺杂 | 增强材料表面结构稳定性, 抑制材料表面发生的相转变和晶格氧流失, 缓解电压衰减问题. | 可选择的掺杂离子种类较少, 离子在表层的扩散深度不容易控制.直接对成品进行表面掺杂, 容易增大材料表面的晶格缺陷. | Na+掺杂进入Li层, 在层间起到支柱的作用. 25 mA?g-1下, 材料首放和首库提升至286 mAh?g-1和87%. | [ | ||
| Ru4+取代了材料表面的部分Mn4+, 在0.1 C下, 首放280 mAh?g-1. 0.1 C下循环100次后, 容量保持率为98.1%. | [ | |||||
| 表面化学处理 | 活化材料表面Li2MnO3相, 减少材料的首次不可逆容量, 提高材料的首次库伦效率. | 处理后对材料表面结构会造成破坏, 会降低材料的循环稳定性.用气体处理材料比液体处理的反应温和, 对材料表面结构影响也较小. | (NH4)2SO4溶液处理材料, 300 mA?g-1下首放可达270 mAh?g-1, 首库从原来的76%提升至95%. | [ | ||
| CO2与材料发生气固界面反应, 在材料表面形成了均匀的一层氧空位.首放301 mAh?g-1, 首库高达93.8%. | [ | |||||
| 表面联合改性 | 避免了单一表面改性方式的不足, 将会成为富锂锰基正极材料的表面改性研究的一个趋势. | 涉及的工艺步骤较多, 且对两种以上表面改性方法的协同改性机理的认识还不足, 需要进一步去研究优化和简化现有方法. | Li2SnO3包覆和表面Sn4+掺杂材料. 1 wt%包覆量, 在5 C下放电比容量126 mAh?g-1, 1 C, 55 ℃下循环70次, 放电比容量保持在235.1 mAh?g-1. | [ | ||
| [1] |
Goodenough J. B. Energy Storage Mater. 2015, 1, 158
doi: 10.1016/j.ensm.2015.07.001 |
| [2] |
Kalluri S.; Yoon M.; Jo M.; Park S.; Myeong S.; Kim J.; Dou S. X.; Guo Z.; Cho J. Adv. Energy Mater. 2017, 7, 1601507
doi: 10.1002/aenm.201601507 |
| [3] |
Deng B.; Sun W.; Wang H.; Chen T.; Li X.; Qu M.; Peng G. Acta Chim. Sinica 2018, 76, 259
doi: 10.6023/A17110517 |
|
邓 邦为; 孙 万琦; 王 昊; 陈 滔; 李 璇; 瞿 美臻; 彭 工厂 化学学报 2018, 76, 259
doi: 10.6023/A17110517 |
|
| [4] |
Zheng Z.; Wu Z.; Xiang W.; Guo X. Acta Chim. Sinica 2017, 75, 501
doi: 10.11862/CJIC.2017.053 |
|
郑 卓; 吴 振国; 向 伟; 郭 孝东 化学学报 2017, 75, 501
doi: 10.11862/CJIC.2017.053 |
|
| [5] |
Hua W.; Wang Y.; Zhong Y.; Wang G.; Zhong B.; Fang B.; Guo X.; Liao S.; Wang H. Chin. J. Chem. 2015, 33, 261
doi: 10.1002/cjoc.201400551 |
| [6] |
Wen L.; Pilgun O.; Xien L.; Min-Joon L.; Woongrae C.; Sujong C.; Youngsik K.; Jaephil C. Angew. Chem. 2015, 54, 4440
doi: 10.1002/anie.201409262 |
| [7] |
Hou P.; Yin J.; Ding M.; Huang J.; Xu X. Small 2017, 13, 1701802
doi: 10.1002/smll.201701802 |
| [8] |
Manthiram A.; Knight J. C.; Myung S. T.; Oh S. M.; Sun Y. K. Adv. Energy Mater. 2016, 6, 1501010
doi: 10.1002/aenm.201501010 |
| [9] |
Erickson E. M.; Schipper F.; Penki T. R.; Shin J. Y.; Erk C.; Chesneau F. F.; Markovsky B.; Aurbach D. J. Electrochem. Soc. 2017, 164, A6220
doi: 10.1149/2.0351701jes |
| [10] |
Kim J.; Lee H.; Cha H.; Yoon M.; Park M.; Cho J. Adv. Energy Mater. 2018, 8, 1702028
doi: 10.1002/aenm.201702028 |
| [11] | Numata K.; Sakaki C.; Yamanaka S. Chem. Lett. 1997, 1997, 725 |
| [12] |
Numata K.; Sakaki C.; Yamanaka S. Solid State Ionics 1999, 117, 257
doi: 10.1016/S0167-2738(98)00417-2 |
| [13] |
Lu Z. H.; Macneil D. D.; Dahn J. R. Electrochem. Solid-State Lett. 2001, 7, A503
doi: 10.1149/1.1819867 |
| [14] |
Johnson C. S.; Kim J. S.; Lefief C.; Li N.; Vaughey J. T.; Thackeray M. M. Electrochem. Commun. 2004, 6, 1085
doi: 10.1016/j.elecom.2004.08.002 |
| [15] |
Thackeray M. M.; Johnson C. S.; Vaughey J. T.; Li N.; Hackney S. A. J. Mater. Chem. 2005, 15, 2257
doi: 10.1039/b417616m |
| [16] |
Thackeray M. M.; Kang S. H.; Johnson C. S.; Vaughey J. T.; Benedek R.; Hackney S. A. J. Mater. Chem. 2007, 17, 3112
doi: 10.1039/b702425h |
| [17] |
Wang Z.; Yin Y.; Ren Y.; Wang Z.; Gao M.; Ma T.; Zhuang W.; Lu S.; Fan A.; Amine K. Nano Energy 2017, 31, 247
doi: 10.1016/j.nanoen.2016.10.014 |
| [18] |
Nayak P. K.; Erickson E. M.; Schipper F.; Penki T. R.; Munichandraiah N.; Adelhelm P.; Sclar H.; Amalraj F.; Markovsky B.; Aurbach D. Adv. Energy Mater. 2018, 8, 1702397
doi: 10.1002/aenm.201702397 |
| [19] |
Li M.; Lu J.; Chen Z.; Amine K. Adv. Mater. 2018, 30, 1800561
doi: 10.1002/adma.201800561 |
| [20] |
Zuo Y.; Li B.; Jiang N.; Chu W.; Zhang H.; Zou R.; Xia D. Adv. Mater. 2018, 30, 1707255
doi: 10.1002/adma.201707255 |
| [21] |
Zheng J.; Myeong S.; Cho W.; Yan P.; Xiao J.; Wang C.; Cho J.; Zhang J. G. Adv. Energy Mater. 2016, 7, 1601284
doi: 10.1002/aenm.v7.6 |
| [22] |
Assat G.; Tarascon J. -M. Nat. Energy 2018, 3, 373
doi: 10.1038/s41560-018-0097-0 |
| [23] |
Yang C.; Gong Z.; Zhao W.; Yang Y. Acta Chim. Sinica 2017, 75, 212
doi: 10.7503/cjcu20160458 |
|
杨 春; 龚 正良; 赵 文高; 杨 勇 化学学报 2017, 75, 212
doi: 10.7503/cjcu20160458 |
|
| [24] |
Gauthier M.; Carney T. J.; Grimaud A.; Giordano L.; Pour N.; Chang H. H.; Fenning D. P.; Lux S. F.; Paschos O.; Bauer C. J. Phys. Chem. Lett. 2015, 6, 4653
doi: 10.1021/acs.jpclett.5b01727 |
| [25] |
Xu B.; Fell C. R.; Chi M.; Meng Y. S. Energy Environ. Sci. 2011, 4, 2223
doi: 10.1039/c1ee01131f |
| [26] |
Oh P.; Ko M.; Myeong S.; Kim Y.; Cho J. Adv. Energy Mater. 2014, 4, 1400631
doi: 10.1002/aenm.201400631 |
| [27] |
Yan P.; Nie A.; Zheng J.; Zhou Y.; Lu D.; Zhang X.; Xu R.; Belharouak I.; Zu X.; Xiao J. Nano Lett. 2015, 15, 514
doi: 10.1021/nl5038598 |
| [28] |
Croy J. R.; Balasubramanian M.; Gallagher K. G.; Burrell A. K. Acc. Chem. Res. 2015, 48, 2813
doi: 10.1021/acs.accounts.5b00277 |
| [29] |
Kim J.-S.; Johnson C. S.; Vaughey J. T.; Thackeray M. M.; Hackney S. A.; Yoon W.; Grey C. P. Chem. Mater. 2004, 16, 1996
doi: 10.1021/cm0306461 |
| [30] |
Kang S. H.; Kempgens P.; Greenbaum S.; Kropf A. J.; Amine K.; Thackeray M. M. J. Mater. Chem. 2007, 17, 2069
doi: 10.1039/B618715C |
| [31] |
Jarvis K. A.; Deng Z.; Allard L. F.; Manthiram A.; Ferreira P. J. Chem. Mater. 2011, 23, 3614
doi: 10.1021/cm200831c |
| [32] |
Mccalla E.; Lowartz C. M.; Brown C. R.; Dahn J. R. Chem. Mater. 2013, 25, 912
doi: 10.1021/cm304002b |
| [33] |
Shunmugasundaram R.; Senthil Arumugam R.; Dahn J. R. Chem. Mater. 2015, 27, 757
doi: 10.1021/cm504583y |
| [34] |
Shunmugasundaram R.; Senthil Arumugam R.; Harris K. J.; Goward G. R.; Dahn J. R. Chem. Mater. 2016, 28, 55
doi: 10.1021/acs.chemmater.5b02104 |
| [35] |
Zheng J.; Xu P.; Gu M.; Xiao J.; Browning N. D.; Yan P.; Wang C.; Zhang J. -G. Chem. Mater. 2015, 27, 1381
doi: 10.1021/cm5045978 |
| [36] |
Gu M.; Belharouak I.; Zheng J.; Wu H.; Xiao J.; Genc A.; Amine K.; Thevuthasan S.; Baer D. R.; Zhang J. -G. ACS Nano 2013, 7, 760
doi: 10.1021/nn305065u |
| [37] |
Hong J.; Seo D. H.; Kim S. W.; Gwon H.; Oh S. T.; Kang K. J. Mater. Chem. 2010, 20, 10179
doi: 10.1039/c0jm01971b |
| [38] |
Xiao B.; Sun X. Adv. Energy Mater. 2018, 8, 1802057
doi: 10.1002/aenm.201802057 |
| [39] |
Hu E.; Yu X.; Lin R.; Bi X.; Lu J.; Bak S.; Nam K.-W.; Xin H. L.; Jaye C.; Fischer D. A.; Amine K.; Yang X. -Q. Nat. Energy 2018, 3, 690
doi: 10.1038/s41560-018-0207-z |
| [40] |
Dai K.; Wu J.; Zhuo Z.; Li Q.; Sallis S.; Mao J.; Ai G.; Sun C.; Li Z.; Gent W. E.; Chueh W. C.; Chuang Y.-d.; Zeng R.; Shen Z.-x.; Pan F.; Yan S.; Piper L. F. J.; Hussain Z.; Liu G.; Yang W. Joule 2019, 3, 518
doi: 10.1016/j.joule.2018.11.014 |
| [41] |
Zheng J.; Myeong S.; Cho W.; Yan P.; Xiao J.; Wang C.; Cho J.; Zhang J. G. Adv. Energy Mater. 2017, 7, 1601284
doi: 10.1002/aenm.201601284 |
| [42] |
Shi S. J.; Tu J. P.; Tang Y. Y.; Liu X. Y.; Zhang Y. Q.; Wang X. L.; Gu C. D. Electrochim. Acta 2013, 88, 671
doi: 10.1016/j.electacta.2012.10.111 |
| [43] |
Han E.; Li Y.; Zhu L.; Zhao L. Solid State Ionics 2014, 255, 113
doi: 10.1016/j.ssi.2013.12.018 |
| [44] |
Yan P.; Zheng J.; Zhang X.; Xu R.; Amine K.; Xiao J.; Zhang J.-G.; Wang C. -M. Chem. Mater. 2016, 28, 857
doi: 10.1021/acs.chemmater.5b04301 |
| [45] |
Kobayashi G.; Irii Y.; Matsumoto F.; Ito A.; Ohsawa Y.; Yamamoto S.; Cui Y.; Son J. Y.; Sato Y. J. Power Sources 2016, 303, 250
doi: 10.1016/j.jpowsour.2015.11.014 |
| [46] |
Lee G.-H.; Choi I. H.; Oh M. Y.; Park S. H.; Nahm K. S.; Aravindan V.; Lee Y. -S. Electrochim. Acta 2016, 194, 454
doi: 10.1016/j.electacta.2016.02.129 |
| [47] |
Xie Y.; Chen S.; Lin Z.; Yang W.; Zou H.; Sun R. W. -Y. Electrochem. Commun. 2019, 99, 65
doi: 10.1016/j.elecom.2019.01.005 |
| [48] |
Mu K.; Cao Y.; Hu G.; Du K.; Yang H.; Gan Z.; Peng Z. Electrochim. Acta 2018, 273, 88
doi: 10.1016/j.electacta.2018.04.027 |
| [49] |
Chen C.; Geng T.; Du C.; Zuo P.; Cheng X.; Ma Y.; Yin G. J. Power Sources 2016, 331, 91
doi: 10.1016/j.jpowsour.2016.09.051 |
| [50] |
Rastgoo-Deylami M.; Javanbakht M.; Omidvar H. Solid State Ionics 2019, 331, 74
doi: 10.1016/j.ssi.2018.12.025 |
| [51] |
Zheng J. M.; Zhang Z. R.; Wu X. B.; Dong Z. X.; Zhu Z.; Yang Y. J. Electrochem. Soc. 2008, 155, A775
doi: 10.1149/1.2966694 |
| [52] |
Zheng J.; Gu M.; Xiao J.; Polzin B. J.; Yan P.; Chen X.; Wang C.; Zhang J. -G. Chem. Mater. 2014, 26, 6320
doi: 10.1021/cm502071h |
| [53] |
Pang S.; Wang Y.; Chen T.; Shen X.; Xi X.; Liao D. Ceram. Int. 2016, 42, 5397
doi: 10.1016/j.ceramint.2015.12.076 |
| [54] |
Hu G.; Qi X.; Hu K.; Lai X.; Zhang X.; Du K.; Peng Z.; Cao Y. Electrochim. Acta 2018, 265, 391
doi: 10.1016/j.electacta.2018.01.176 |
| [55] |
Sun S.; Wan N.; Wu Q.; Zhang X.; Pan D.; Bai Y.; Lu X. Solid State Ionics 2015, 278, 85
doi: 10.1016/j.ssi.2015.05.021 |
| [56] |
Liu X.; Huang T.; Yu A. Electrochim. Acta 2015, 163, 82
doi: 10.1016/j.electacta.2015.02.155 |
| [57] |
Liu H.; Qian D.; Verde M. G.; Zhang M.; Baggetto L.; An K.; Chen Y.; Carroll K. J.; Lau D.; Chi M.; Veith G. M.; Meng Y. S. ACS Appl. Mater. Interfaces 2015, 7, 19189
doi: 10.1021/acsami.5b04932 |
| [58] |
Lu C.; Wu H.; Zhang Y.; Liu H.; Chen B.; Wu N.; Wang S. J. Power Sources 2014, 267, 682
doi: 10.1016/j.jpowsour.2014.05.122 |
| [59] |
Wu Y.; Murugan A. V.; Manthiram A. J. Electrochem. Soc. 2008, 155, A635
doi: 10.1149/1.2948350 |
| [60] |
Ma J.; Li B.; An L.; Wei H.; Wang X.; Yu P.; Xia D. J. Power Sources 2015, 277, 393
doi: 10.1016/j.jpowsour.2014.11.133 |
| [61] |
Xiao B.; Wang B.; Liu J.; Kaliyappan K.; Sun Q.; Liu Y.; Dadheech G.; Balogh M. P.; Yang L.; Sham T. -K. Nano Energy 2017, 34, 120
doi: 10.1016/j.nanoen.2017.02.015 |
| [62] |
Xie Q.; Zhao C.; Hu Z.; Huang Q.; Chen C.; Liu K. RSC Adv. 2015, 5, 77324
doi: 10.1039/C5RA13233A |
| [63] |
Chen J.; Li Z.; Xiang H.; Wu W.; Cheng S.; Zhang L.; Wang Q.; Wu Y. RSC Adv. 2015, 5, 3031
doi: 10.1039/C4RA11370E |
| [64] |
Wu F.; Li N.; Su Y.; Lu H.; Zhang L.; An R.; Wang Z.; Bao L.; Chen S. J. Mater. Chem. 2012, 22, 1489
doi: 10.1039/C1JM14459F |
| [65] |
Liu Y.; Liu S.; Wang Y.; Chen L.; Chen X. J. Power Sources 2013, 222, 455
doi: 10.1016/j.jpowsour.2012.09.014 |
| [66] |
Guo S.; Yu H.; Liu P.; Liu X.; Li D.; Chen M.; Ishida M.; Zhou H. J. Mater. Chem. A 2014, 2, 4422
doi: 10.1039/c3ta15206e |
| [67] |
Jin Y.; Xu Y.; Sun X.; Xiong L.; Mao S. Appl. Surf. Sci. 2016, 384, 125
doi: 10.1016/j.apsusc.2016.04.136 |
| [68] |
He H.; Zan L.; Zhang Y. J. Alloys Compd. 2016, 680, 95
doi: 10.1016/j.jallcom.2016.04.115 |
| [69] |
Jin Y.; Xu Y.; Xiong L.; Sun X.; Li L.; Li L. Solid State Ionics 2017, 310, 62
doi: 10.1016/j.ssi.2017.07.012 |
| [70] |
Li Y.; Huang H.; Yu J.; Xia Y.; Liang C.; Gan Y.; Zhang J.; Zhang W. J. Alloys Compd. 2019, 783, 349
doi: 10.1016/j.jallcom.2018.12.357 |
| [71] |
Wang Z.; Liu E.; He C.; Shi C.; Li J.; Zhao N. J. Power Sources 2013, 236, 25
doi: 10.1016/j.jpowsour.2013.02.022 |
| [72] |
Wang Z.; Lu H.-Q.; Yin Y.-P.; Sun X.-Y.; Bai X.-T.; Shen X.-L.; Zhuang W.-D.; Lu S. -G. Rare Metals 2017, 36, 899
doi: 10.1007/s12598-015-0647-6 |
| [73] |
Zhao T.; Li L.; Chen R.; Wu H.; Zhang X.; Chen S.; Xie M.; Wu F.; Lu J.; Amine K. Nano Energy 2015, 15, 164
doi: 10.1016/j.nanoen.2015.04.013 |
| [74] |
Bian X.; Fu Q.; Bie X.; Yang P.; Qiu H.; Pang Q.; Chen G.; Du F.; Wei Y. Electrochim. Acta 2015, 174, 875
doi: 10.1016/j.electacta.2015.06.085 |
| [75] |
Chen D.; Zheng F.; Li L.; Chen M.; Zhong X.; Li W.; Lu L. J. Power Sources 2017, 341, 147
doi: 10.1016/j.jpowsour.2016.11.020 |
| [76] |
Lee Y.; Lee J.; Lee K. Y.; Mun J.; Lee J. K.; Choi W. J. Power Sources 2016, 315, 284
doi: 10.1016/j.jpowsour.2016.03.024 |
| [77] |
Zhou L.; Yin Z.; Tian H.; Ding Z.; Li X.; Wang Z.; Guo H. Appl. Surf. Sci. 2018, 456, 763
doi: 10.1016/j.apsusc.2018.06.114 |
| [78] |
Martha S. K.; Nanda J.; Kim Y.; Unocic R. R.; Pannala S.; Dudney N. J. J. Mater. Chem. A 2013, 1, 5587
doi: 10.1039/c3ta10586e |
| [79] |
Kang S. H.; Thackeray M. M. Electrochem. Commun. 2009, 11, 748
doi: 10.1016/j.elecom.2009.01.025 |
| [80] |
Qiao Q. Q.; Zhang H. Z.; Li G. R.; Ye S. H.; Wang C. W.; Gao X. P. J. Mater. Chem. A 2013, 1, 5262
doi: 10.1039/c3ta00028a |
| [81] |
Zheng F.; Yang C.; Xiong X.; Xiong J.; Hu R.; Chen Y.; Liu M. Angew. Chem. Int. Ed. 2015, 54, 13058
doi: 10.1002/anie.201506408 |
| [82] |
Chen Y.; Xie K.; Zheng C.; Ma Z.; Chen Z. ACS Appl. Mater. Interfaces 2014, 6, 16888
doi: 10.1021/am504412n |
| [83] |
Li H.; Zhou H. Chem. Commun. 2012, 48, 1201
doi: 10.1039/C1CC14764A |
| [84] |
Xia Q.; Zhao X.; Xu M.; Ding Z.; Liu J.; Chen L.; Ivey D. G.; Wei W. J. Mater. Chem. A 2015, 3, 3995
doi: 10.1039/C4TA05848H |
| [85] |
Pang S.; Xu K.; Wang Y.; Shen X.; Wang W.; Su Y.; Zhu M.; Xi X. J. Power Sources 2017, 365, 68
doi: 10.1016/j.jpowsour.2017.08.077 |
| [86] |
Park K.; Kim J.; Park J.-H.; Hwang Y.; Han D. J. Power Sources 2018, 408, 105
doi: 10.1016/j.jpowsour.2018.10.001 |
| [87] |
Ma Y.; Liu P.; Xie Q.; Zhang G.; Zheng H.; Cai Y.; Li Z.; Wang L.; Zhu Z.-Z.; Mai L. Nano Energy 2019, 59, 184
doi: 10.1016/j.nanoen.2019.02.040 |
| [88] |
Wu F.; Li N.; Su Y.; Shou H.; Bao L.; Yang W.; Zhang L.; An R.; Chen S. Adv. Mater. 2013, 25, 3722
doi: 10.1002/adma.201300598 |
| [89] |
Wang L.; Zhao D.; Liu X.; Yu P.; Fu H. Acta Chim. Sinica 2017, 75, 231
doi: 10.7503/cjcu20160577 |
|
王 蕾; 赵 东东; 刘 旭; 于 鹏; 付 宏刚 化学学报 2017, 75, 231
doi: 10.7503/cjcu20160577 |
|
| [90] |
Li Z.; Wang Z.; Li Q.; Ban L.; Zhuang W.; Lu S. Chinese J. Inorg. Chem. 2019, 35, 1561
doi: 10.11862/CJIC.2019.192 |
|
李 钊; 王 忠; 李 强; 班 丽卿; 庄 卫东; 卢 世刚 无机化学学报 2019, 35, 1561
doi: 10.11862/CJIC.2019.192 |
|
| [91] |
Song B.; Lai M. O.; Liu Z.; Liu H.; Lu L. J. Mater. Chem. A 2013, 1, 9954
doi: 10.1039/c3ta11580a |
| [92] |
Zhang J.; Lu Q.; Fang J.; Wang J.; Yang J.; Nuli Y. ACS Appl. Mater. Interfaces 2014, 6, 17965
doi: 10.1021/am504796n |
| [93] |
Wu C.; Fang X.; Guo X.; Mao Y.; Ma J.; Zhao C.; Wang Z.; Chen L. J. Power Sources 2013, 231, 44
doi: 10.1016/j.jpowsour.2012.11.138 |
| [94] |
Wang D.; Wang X.; Yang X.; Yu R.; Ge L.; Shu H. J. Power Sources 2015, 293, 89
doi: 10.1016/j.jpowsour.2015.05.058 |
| [95] |
Wu F.; Liu J.; Li L.; Zhang X.; Luo R.; Ye Y.; Chen R. ACS Appl. Mater. Interfaces 2016, 8, 23095
doi: 10.1021/acsami.6b07431 |
| [96] |
Kim I. T.; Knight J. C.; Celio H.; Manthiram A. J. Mater. Chem. A 2014, 2, 8696
doi: 10.1039/c4ta00898g |
| [97] |
Chen D.; Tu W.; Chen M.; Hong P.; Zhong X.; Zhu Y.; Yu Q.; Li W. Electrochim. Acta 2016, 193, 45
doi: 10.1016/j.electacta.2016.02.043 |
| [98] |
Liu H.; Chen C.; Du C.; He X.; Yin G.; Song B.; Zuo P.; Cheng X.; Ma Y.; Gao Y. J. Mater. Chem. A 2015, 3, 2634
doi: 10.1039/C4TA04823G |
| [99] |
Nayak P. K.; Grinblat J.; Levi M.; Levi E.; Kim S.; Choi J. W.; Aurbach D. Adv. Energy Mater. 2016, 6, 1502398
doi: 10.1002/aenm.201502398 |
| [100] |
Su X.; Wang X.; Chen H.; Yu Z.; Qi J.; Tao S.; Chu W.; Song L. Chin. J. Chem. 2017, 35, 1853
doi: 10.1002/cjoc.201700265 |
| [101] |
Qing R. P.; Shi J. L.; Xiao D. D.; Zhang X. D.; Yin Y. X.; Zhai Y. B.; Gu L.; Guo Y. G. Adv. Energy Mater. 2016, 6, 1501914
doi: 10.1002/aenm.201501914 |
| [102] |
Liu S.; Liu Z.; Shen X.; Li W.; Gao Y.; Banis M. N.; Li M.; Chen K.; Zhu L.; Yu R. Adv. Energy Mater. 2018, 8, 1802105
doi: 10.1002/aenm.201802105 |
| [103] |
Zhang X.; Cao S.; Yu R.; Li C.; Huang Y.; Wang Y.; Wang X.; Gairong C. ACS Appl. Energy Mater. 2019, 2, 1563
doi: 10.1021/acsaem.8b02178 |
| [104] |
Huang J.; Liu H.; Hu T.; Meng Y. S.; Luo J. J. Power Sources 2018, 375, 21
doi: 10.1016/j.jpowsour.2017.11.048 |
| [105] |
Zhao Y.; Liu J.; Wang S.; Ji R.; Xia Q.; Ding Z.; Wei W.; Liu Y.; Wang P.; Ivey D. G. Adv. Funct. Mater. 2016, 26, 4760
doi: 10.1002/adfm.201600576 |
| [106] |
Shang H.; Ning F.; Li B.; Zuo Y.; Lu S.; Xia D. ACS Appl. Mater. Interfaces 2018, 10, 21349
doi: 10.1021/acsami.8b06271 |
| [107] | Kang S. H.; Johnson C. S.; Vaughey J. T.; Amine K.; Thackeray M. M. J. Electrochem. Soc. 2006 153 |
| [108] |
Paik Y.; Grey C. P.; Johnson C. S.; Kim J.-S.; Thackeray M. M. Chem. Mater. 2002, 14, 5109
doi: 10.1021/cm0206385 |
| [109] |
Benedek R.; Thackeray M. M.; Van De Walle A. Chem. Mater. 2008, 20, 5485
doi: 10.1021/cm703042r |
| [110] |
Denis Y.; Yanagida K.; Nakamura H. J. Electrochem. Soc. 2010, 157, A1177
doi: 10.1149/1.3479382 |
| [111] |
Oh P.; Myeong S.; Cho W.; Lee M.-J.; Ko M.; Jeong H. Y.; Cho J. Nano Lett. 2014, 14, 5965
doi: 10.1021/nl502980k |
| [112] |
Zhang J.; Lei Z.; Wang J.; Nuli Y.; Yang J. ACS Appl. Mater. Interfaces 2015, 7, 15821
doi: 10.1021/acsami.5b02937 |
| [113] |
Erickson E. M.; Sclar H.; Schipper F.; Liu J.; Tian R.; Ghanty C.; Burstein L.; Leifer N.; Grinblat J.; Talianker M. Adv. Energy Mater. 2017, 7, 1700708
doi: 10.1002/aenm.201700708 |
| [114] |
Qiu B.; Zhang M.; Wu L.; Wang J.; Xia Y.; Qian D.; Liu H.; Hy S.; Chen Y.; An K.; Zhu Y.; Liu Z.; Meng Y. S. Nat. Commun. 2016, 7, 12108
doi: 10.1038/ncomms12108 |
| [115] |
Danna Q.; Bo X.; Miaofang C.; Shirley M. Y. Phys. Chem. Chem. Phys. 2014, 16, 14665
doi: 10.1039/C4CP01799D |
| [116] |
James C.; Wu Y.; Sheldon B. W.; Qi Y. Solid State Ionics 2016, 289, 87
doi: 10.1016/j.ssi.2016.02.019 |
| [117] |
Huang Z.; Xiong T.; Lin X.; Tian M.; Zeng W.; He J.; Shi M.; Li J.; Zhang G.; Mai L. J. Power Sources 2019, 432, 8
doi: 10.1016/j.jpowsour.2019.05.069 |
| [118] |
Liu W.; Oh P.; Liu X.; Myeong S.; Cho W.; Cho J. Adv. Energy Mater. 2015, 5, 1500274
doi: 10.1002/aenm.201500274 |
| [119] |
Zhang X. D.; Shi J. L.; Liang J. Y.; Yin Y. X.; Zhang J. N.; Yu X. Q.; Guo Y. G. Adv. Mater. 2018, 30, 1801751
doi: 10.1002/adma.201801751 |
| [120] |
Cui H.; Li H.; Liu J.; Zhang Y.; Cheng F.; Chen J. Inorg. Chem. Front. 2019, 6, 1694
doi: 10.1039/C9QI00333A |
| [121] |
Guo H.; Jia K.; Han S.; Zhao H.; Qiu B.; Xia Y.; Liu Z. Adv. Mater. Interfaces 2018, 5, 1701465
doi: 10.1002/admi.201701465 |
| [122] |
Li Q.; Zhou D.; Zhang L.; Ning D.; Chen Z.; Xu Z.; Gao R.; Liu X.; Xie D.; Schumacher G. Adv. Funct. Mater. 2019, 29, 1806706
doi: 10.1002/adfm.201806706 |
| [1] | 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益. 磷酸锂原位包覆富锂锰基锂离子电池正极材料[J]. 化学学报, 2020, 78(12): 1426-1433. |
| [2] | 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心. 铝掺杂及钨酸锂表面包覆双效提升富锂锰基正极材料的循环稳定性[J]. 化学学报, 2020, 78(11): 1268-1274. |
| [3] | 吴晓春,汤国庆,张桂兰,邹炳锁,余保龙,陈文驹. 不同制备条件对纳米Bi~2O~3发光的影响[J]. 化学学报, 1996, 54(2): 146-151. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||