化学学报 ›› 2014, Vol. 72 ›› Issue (7): 778-797.DOI: 10.6023/A14050364 上一篇 下一篇
所属专题: 不对称催化与合成; 纪念南开大学化学学科创建100周年
综述
谢建华, 周其林
投稿日期:2014-05-08
发布日期:2014-06-05
通讯作者:
谢建华, 周其林
E-mail:jhxie@nankai.edu.cn;qlzhou@nankai.edu.cn
基金资助:Xie Jianhua, Zhou Qilin
Received:2014-05-08
Published:2014-06-05
Supported by:文章分享
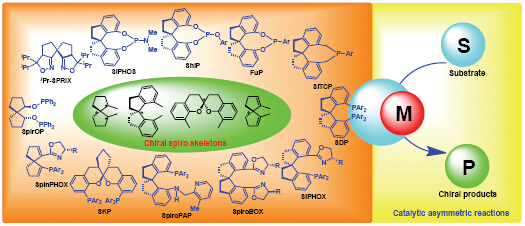
过渡金属参与的不对称催化反应是有机合成化学研究的前沿和热点. 寻找和发现新颖配体骨架并开展新型高效的手性配体及催化剂的设计合成是不对称催化反应研究的核心内容. 从20世纪90年代,特别是进入21世纪以来,螺环骨架手性配体受到了广泛的关注,并逐渐发展成为特色鲜明的手性配体类别. 手性螺环配体的骨架已由多手性的螺[4.4]壬烷骨架发展到只具有单一手性的螺二氢茚和螺[4.4]壬二烯等螺环骨架类型,形成了包括手性螺环单磷配体、双膦配体、膦氮配体、双氮配体等丰富的手性配体库. 这些手性螺环配体及其催化剂不仅在不对称催化氢化、不对称碳―碳键形成、不对称碳―杂原子键形成等多种类型的不对称催化反应中均表现出优异的催化活性和对映选择性,且使得许多原先难以控制对映选择性的不对称催化反应变得可能. 而今,手性螺环结构已成为“优势结构”,相应的手性螺环配体及其催化剂已被国内外同行广泛采用. 手性螺环配体的兴起为手性催化剂研究增加了活力,极大地促进了不对称合成化学的发展. 今后,手性螺环配体的研究除了将向新型、高效、高选择性手性配体及催化剂方向发展外,将其应用于新的不对称催化反应的对映选择性控制、以及应用于手性天然产物和药物的高效不对称合成将成为新的研究热点.
谢建华, 周其林. 神奇的手性螺环配体[J]. 化学学报, 2014, 72(7): 778-797.
Xie Jianhua, Zhou Qilin. Magical Chiral Spiro Ligands[J]. Acta Chimica Sinica, 2014, 72(7): 778-797.
| [1] (a) Lin, G.-Q.; Li, Y.-M.; Chan, A. S. C. Priciples and Applications of Asymmetric Synthesis, Wiley, New York, 2001.(b) Ed.: Ojima, I., Catalytic Asymmetric Synthesis, Wiley-VCH, New York, 2000.(c) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2012, 70, 1427 (谢建华, 周其林, 化学学报, 2012, 70, 1427).(d) Liu, Y.; Wang, Z.; Ding, K.-L. Acta Chim. Sinica 2012, 70, 1446 (刘龑, 王正, 丁奎岭, 化学学报, 2012, 70, 1446).(e) Zheng, K.; Lin, L.-L.; Feng, X.-M. Acta Chim. Sinica 2012, 70, 1758 (郑柯, 林丽丽, 冯小明, 化学学报, 2012, 70, 1758).[2] (a) Knowles, W. S.; Sabacky, M. J. J. Chem. Soc., Chem. Commun. 1968, 1445.(b) Horner, L.; Siegel, H.; Büthe, H. Angew. Chem., Int. Ed. 1968, 7, 942.[3] (a) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.(b) Ed.: Zhou, Q.-L., Privileged Chiral Ligands and Catalysts, Wiley-VCH Verlag GmbH & Co. KgaA, 2011.[4] Knowles, W. S.; Sabacky, M. J.; Vineyard, B. D. J. Chem. Soc., Chem. Commun. 1972, 10.[5] (a) Dang, T. P.; Kagan, H. G. J. Chem. Soc., Chem. Commun. 1972, 481.(b) Dang, T. P.; Kagan, H. G. J. Am. Chem. Soc. 1972, 94, 6429.[6] Knowles. W. S. Acc. Chem. Res. 1983, 16, 106.[7] Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932.[8] Noyori, R. Angew. Chem., Int. Ed. 2002, 41, 2008.[9] (a) Zhu, S. F.; Zhou, Q.-L. In Privileged Chiral Ligands and Catalysts, Ed.: Zhou, Q.-L., Wiley-VCH Verlag GmbH & Co. KgaA, 2011, pp. 137~170.(b) Zhou, Q.-L.; Xie, J.-H. Top. Organomet. Chem. 2011, 36, 1.(c) Ding, K.; Han, Z.; Wang, Z. Chem.-Asian J. 2009, 4, 32.(d) Xie, J.-H.; Zhou, Q.-L. Acc. Chem. Res. 2008, 41, 581.(e) Zhang, Z.-H. Chin. J. Org. Chem. 2005, 25, 355. (张占辉, 有机化学, 2005, 25, 355.)[10] Srivastava, N.; Mital, A.; Kumar, A. Chem. Commun. 1992, 493.[11] Chan, A. S. C.; Hu, W.-H.; Pai, C.-C.; Lau, C.-P.; Jiang, Y.-Z.; Mi, A.-Q.; Yan, M.; Sun, J.; Lou, R.-L.; Deng, J.-G. J. Am. Chem. Soc. 1997, 119, 9570.[12] (a) Arai, M. A.; Arai, T.; Sasai, H. Org. Lett. 1999, 1, 1795.(b) Arai, M. A.; Kuraishi, M.; Arai, T.; Sasai, H. J. Am. Chem. Soc. 2001, 123, 2907.[13] Birman, V. B.; Rheingold, A. L.; Lam, K.-C. Tetrahedron: Asymmetry 1999, 1, 1975.[14] (a) Hu, A.-G.; Fu, Y.; Xie, J.-H.; Zhou, H.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2002, 41, 2348.(b) Fu, Y.; Xie, J.-H.; Hu, A.-G.; Zhou, H.; Wang, L.-X.; Zhou, Q.-L. Chem. Commun. 2002, 480.[15] Xie, J.-H.; Wang, L.-X.; Fu, Y.; Zhu, S.-F.; Fan, B.-M.; Duan, H.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2003, 125, 4404.[16] Zhu, G.; Cao, P.; Jiang, Q.; Zhang, X. J. Am. Chem. Soc. 1997, 119, 1799.[17] Han, Z.; Wang, Z.; Zhang, X.; Ding, K. Angew. Chem., Int. Ed. 2009, 48, 5345.[18] (a) Jiang, Y.; Xue, S.; Li, Z.; Deng, J.; Mi, A.; Chan, A. S. C. Tetrahedron: Asymmetry 1998, 9, 3185.(b) Jiang, Y.; Xue, S.; Yu, K.; Li, Z.; Deng, J.; Mi, A.; Chan, A. S. C. J. Organomet. Chem. 1999, 586, 159.[19] Lin, C.-W.; Lin, C.-C.; Lam, L. F.-L.; Au-Yeung, T. T.-L.; Chan, A. S. C. Tetrahedron Lett. 2004, 45, 7379.[20] Guo, Z.-Q.; Guan, X.-Y.; Chen, Z.-Y. Tetrahedron: Asymmetry 2006, 17, 468.[21] Takizawa, S.; Honda, Y.; Arai, M. A.; Kato, T.; Sasai, H. Heterocycles 2003, 60, 2551.[22] Kato, T.; Marubayashi, K.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2004, 15, 3693.[23] Bajracharya, G. B.; Arai, M. A.; Koranne, P. S.; Suzuki, T.; Takizawa, S.; Sasai, H. Bull. Chem. Soc. Jpn. 2009, 82, 285.[24] Tsujihara, T.; Takenaka, K.; Onitsuka, K.; Hatanaka, M.; Sasai, H. J. Am. Chem. Soc. 2009, 131, 3452.[25] Lait, S. M.; Parvez, M.; Keay, B. A. Tetrahedron: Asymmetry 2004, 15, 155.[26] Benoit, W. L.; Parvez, M.; Keay, B. A. Tetrahedron: Asymmetry 2009, 20, 69.[27] (a) Zhu, S.-F.; Xie, J.-H.; Liu, B.; Xing, L.; Zhou, Q.-L. Tetrahedron: Asymmetry 2003, 14, 3219.(b) Fu, Y.; Hou, G.-H.; Xie, J.-H.; Xing, L.; Wang, L.-X.; Zhou, Q.-L. J. Org. Chem. 2004, 69, 8157.(c) Fu, Y.; Guo, X.-X.; Zhu, S.-F.; Hu, A.-G.; Xie, J.-H.; Zhou, Q.-L. J. Org. Chem. 2004, 69, 4648.[28] (a) Hou, G.-H.; Xie, J.-H.; Yan, P.-C.; Zhou, Q.-L. J. Am. Chem. Soc. 2009, 131, 1366.(b) Yan, P.-C.; Xie, J.-H.; Hou, G.-H.; Wang, L.-X.; Zhou, Q.-L. Adv. Synth. Catal. 2009, 351, 3176.[29] Shi, W.-J.; Zhang, Q.; Xie, J.-H.; Zhu, S.-F.; Hou, G.-H.; Zhou, Q.-L. J. Am. Chem. Soc. 2006, 128, 2780.[30] (a) Yang, Y.; Zhu, S.-F.; Duan, H.-F.; Zhou, C.-Y.; Wang, L.-X.; Zhou, Q.-L. J. Am. Chem. Soc. 2007, 129, 2248.(b) Yang, Y.; Zhu, S.-F.; Zhou, C.-Y.; Zhou, Q.-L. J. Am. Chem. Soc. 2008, 130, 14052.[31] Mai, D. N.; Wolfe, J. P. J. Am. Chem. Soc. 2010, 132, 12157.[32] (a) Hopkins, B. A.; Wolfe, J. P. Angew. Chem., Int. Ed. 2012, 51, 9886.(b) Babij, N. R.; Wolfe, J. P. Angew. Chem., Int. Ed. 2013, 52, 9247.(c) Babij, N. R.; Rosen, B. R.; Wolfe, J. P. Org. Lett. 2011, 13, 2932.[33] González, A. Z.; Benitez, D.; Tkatchouk, E.; Goddard, W. A.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 5500.[34] Suárez-Pantiga, S.; Hernández-Díaz, C.; Rubio, E.; González, J. M. Angew. Chem., Int. Ed. 2012, 51, 11552.[35] Coulter, M. M.; Kou, K. G. M.; Galligan, B.; Dong, V. M. J. Am. Chem. Soc. 2010, 132, 16330.[36] Shi, W.-J.; Wang, L.-X.; Fu, Y.; Zhu, S.-F.; Zhou, Q.-L. Tetrahedron: Asymmetry 2003, 14, 3867.[37] (a) Duan, H.-F.; Xie, J.-H.; Shi, W.-J.; Zhang, Q.; Zhou, Q.-L. Org. Lett. 2006, 8, 1479.(b) Duan, H.-F.; Jia, Y.-X.; Wang, L.-X.; Zhou, Q.-L. Org. Lett. 2006, 8, 2567.(c) Duan, H.-F.; Xie, J.-H.; Qiao, X.-C.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2008, 47, 4351.[38] Zhu, S.-F.; Qiao, X.-C.; Zhang, Y.-Z.; Wang, L.-X.; Zhou, Q.-L. Chem. Sci. 2011, 2, 1135.[39] Hou, G.-H.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. J. Am. Chem. Soc. 2006, 128, 11774.[40] Zhu, S.-F.; Yang, Y.; Wang, L.-X.; Liu, B.; Zhou, Q.-L. Org. Lett. 2005, 7, 2333.[41] Zhang, W.; Zhu, S.-F.; Qiao, X.-C.; Zhou, Q.-L. Chem.-Asian J. 2008, 3,2105.[42] Zhou, C.-Y.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. J. Am. Chem. Soc. 2010, 132, 10955.[43] Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2009, 48, 2225.[44] Lundgren, R. J.; Wilsily, A.; Marion, N.; Ma, C.; Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2013, 52, 2525.[45] Xie, J.-H.; Duan, H.-F.; Fan, B.-M.; Cheng, X.; Wang, L.-X.; Zhou, Q.-L. Adv. Synth. Catal. 2006, 346, 625.[46] Fan, B.-M.; Xie, J.-H.; Li, S.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2007, 46, 1275.[47] (a) Zhou, Z.-T.; Xie, J.-H.; Zhou, Q.-L. Adv. Synth. Catal. 2009, 351, 363.(b) Xie, J.-H.; Zhou, Z.-T.; Kong, W.-L.; Zhou, Q.-L. J. Am. Chem. Soc. 2007, 129, 1868.[48] (a) Xie, J.-H.; Liu, S.; Huo, X.-H.; Cheng, X.; Duan, H.-F.; Fan, B.-M.; Wang, L.-X.; Zhou, Q.-L. J. Org. Chem. 2005, 70, 2967.(b) Liu, S.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2007, 46, 7506.(c) Liu, S.; Xie, J.-H.; Li, W.; Kong, W.-L.; Wang, L.-X.; Zhou, Q.-L. Org. Lett. 2009, 11, 4994.(d) Xie, J.-H.; Liu, S.; Kong, W.-L.; Bai, W.-J.; Wang, X.-C.; Wang, L.-X.; Zhou, Q.-L. J. Am. Chem. Soc. 2009, 131, 4222.(e) Bai, W.-J.; Xie, J.-H.; Li, Y.-L.; Liu, S.; Zhou, Q.-L. Adv. Synth. Catal. 2010, 352, 81. (f) Cheng, L.-J.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Adv. Synth. Catal. 2012, 354, 1105. (g) Chen, J.-Q.; Xie, J.-H.; Bao, D.-H.; Liu, S.; Zhou, Q.-L. Org. Lett. 2012, 14, 2714. (h) Cheng, L.-J.; Xie, J.-H.; Chen, Y.; Wang, L.-X.; Zhou, Q.-L. Org. Lett. 2013, 15, 764. (i) Li, G.; Xie, J.-H.; Hou, J.; Zhu, S.-F.; Zhou, Q.-L. Adv. Synth. Catal. 2013, 355, 1597. (j) Liu, C.; Xie, J.-H.; Li, Y.-L.; Chen, J.-Q.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2013, 52, 593.[49] (a) Cheng, X.; Zhang, Q.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2005, 44, 1118.(b) Cheng, X.; Xie, J.-H.; Li, S.; Zhou, Q.-L. Adv. Synth. Catal. 2006, 348, 1271.[50] Tang, W.-J.; Zhu, S.-F.; Xu, L.-J.; Zhou, Q.-L.; Fan, Q.-H.; Zhou, H.-F.; Lam, K.; Chan, A. S. C. Chem. Commun. 2007, 613.[51] (a) Schnider, P.; Koch, G.; Pretot, R.; Wang, G.; Bohnen, M.; Kruger, C.; Pfaltz, A. Chem. Eur. J. 1997, 3, 887.(b) Lightfoot, A.; Schnider, P.; Pfaltz, A. Angew. Chem., Int. Ed. 1998, 37, 2897.(c) Kainz, S.; Brinkmann, A.; Leitner, W.; Pflatz, A. J. Am. Chem. Soc. 1999, 121, 6421.[52] Zhu, S.-F.; Xie, J.-B.; Zhang, Y.-Z.; Li, S.; Zhou, Q.-L. J. Am. Chem. Soc. 2006, 128, 12886.[53] (a) Li, S.; Zhu, S.-F.; Zhang, C.-M.; Song, S.; Zhou, Q.-L. J. Am. Chem. Soc. 2008, 130, 8584.(b) Li, S.; Zhu, S.-F.; Xie, J.-H.; Song, S.; Zhang, C.-M.; Zhou, Q.-L. J. Am. Chem. Soc. 2010, 132, 1172.(c) Song, S.; Zhu, S.-F.; Pu, L.-Y.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2013, 52, 6072.[54] (a) Song, S.; Zhu, S.-F.; Yang, S.; Li, S.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2012, 51, 2708.(b) Song, S.; Zhu, S.-F.; Yu, Y.-B.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2013, 52, 1556.[55] (a) Xie, J.-B.; Xie, J.-H.; Liu, X.-Y.; Kong, W.-L.; Li, S.; Zhou, Q.-L. J. Am. Chem. Soc. 2010, 132, 4538.(b) Xie, J.-B.; Xie, J.-H.; Liu, X.-Y.; Zhang, Q.-Q.; Zhou, Q.-L. Chem.-Asian J. 2011, 6, 899.(c) Zhu, S.-F.; Yu, Y.-B.; Li, S.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2012, 51, 8872.[56] (a) Xie, J.-H.; Liu, X.-Y.; Xie, J.-B.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 7329.(b) Xie, J.-H.; Liu, X.-Y.; Yang, X.-H.; Xie, J.-B.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2012, 51, 201.(c) Yang, X.-H.; Xie, J.-H.; Zhou, Q.-L. Org. Chem. Front. 2014, 1, 190.[57] Yan, P.-C.; Zhu, G.-L.; Xie, J.-H.; Zhang, X.-D.; Zhou, Q.-L.; Li, Y.-Q.; Shen, W.-H.; Che, D.-Q. Org. Process Res. Dev. 2013, 17, 307.[58] Yang, X.-H.; Xie, J.-H.; Liu, W.-P.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2013, 52, 7833.[59] Arai, N.; Ohkuma, T. Chem. Rec. 2012, 12, 284.[60] Liu, B.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. Tetrahedron: Asymmetry 2006, 17, 634.[61] Zhu, S.-F.; Zhou, Q.-L. Acc. Chem. Res. 2012, 45, 1365.[62] (a) Liu, B.; Zhu, S.-F.; Zhang, W.; Chen, C.; Zhou, Q.-L. J. Am. Chem. Soc. 2007, 129, 5834.(b) Zhu, S.-F.; Xu, B.; Wang, G.-P.; Zhou, Q.-L. J. Am. Chem. Soc. 2012, 134, 436.[63] Zhu, S.-F.; Cai, Y.; Mao, H.-X.; Xie, J.-H.; Zhou, Q.-L. Nat. Chem. 2010, 2, 546.[64] Xie, X.-L.; Zhu, S.-F.; Guo, J.-X.; Cai, Y.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2014, 53, 2978.[65] Su, W.; Ma, S. Chem. Commun. 2009, 6198.[66] Shu, W.; Yu, Q.; Ma, S. Adv. Synth. Catal. 2009, 351, 2807.[67] Zhang, Y.-Z.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2008, 47, 8496.[68] Hoffman, T. J.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 10670.[69] (a) Zhang, Y.; Han, Z.; Li, F.; Ding, K.; Zhang, A. Chem. Commun. 2010, 46, 156.(b) Shang, J.; Han, Z.; Li, Y.; Wang, Z.; Ding, K. Chem. Commun. 2012, 48, 5172.(c) Wang, X.; Han, Z.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2012, 51, 936.[70] Han, Z.; Wang, Z.; Zhang, X.; Ding, K. Chin. Sci. Bull. 2010, 55, 2840.[71] Han, Z.; Wang, Z.; Zhang, X.; Ding, K. Scientia Sinica Chimica 2010, 40, 950. (韩召斌, 王正, 张绪穆, 丁奎岭, 中国科学?化学, 2010, 40, 950.)[72] Shimizu, H.; Igarashi, D.; Kuriyama, W.; Yusa, Y.; Sayo, N.; Saito, T. Org. Lett. 2007, 9, 1655.[73] Han, Z.; Wang, Z.; Zhang, X.; Ding, K. Tetrahedron: Asymmetry 2010, 21, 1529.[74] (a) Freixa, Z.; Beentjes, M. S.; Batema, G. D.; Dieleman, C. B.; van Strijdonck, G. P. F.; Reek, J. N. H.; Kamer, P. C. J.; Fraanje, J.; Goubitz, K.; van Leeuwen, P. W. N. M. Angew. Chem., Int. Ed. 2003, 42, 1284.(b) Freixa, Z.; Kamer, P. C. J.; Lutz, M.; Spek, A. L.; van Leeuwen, P. W. N. M. Angew. Chem., Int. Ed. 2005, 44, 4384.[75] Jacquet, O.; Clément, N. D.; Blanco, C.; Belmonte, M. M.; Benet-Buchholz, J.; van Leeuwen, P. W. M. N. Eur. J. Org. Chem. 2012, 4844.[76] (a) Wang, X.; Meng, F.; Wang, Y.; Han, Z.; Chen, Y.-J.; Liu, L.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2012, 51, 9276.(b) Wang, X.; Guo, P.; Wang, X.; Wang, Z.; Ding, K. Adv. Synth. Catal. 2013, 355, 2900.[77] Wang, X.; Guo, P.; Han, Z.; Wang, X.; Wang, Z.; Ding, K. J. Am. Chem. Soc. 2014, 136, 405.[78] Cao, Z.-Y.; Wang, X.; Tan, C.; Zhao, X.-L.; Zhou, J.; Ding, K. J. Am. Chem. Soc. 2013, 135, 8197.[79] Sala, X.; Suárez, E. J. G.; Freixa, Z.; Benet-Buchholz, J.; van Leeuwen, P. W. N. M. Eur. J. Org. Chem. 2008, 6197.[80] Jacquet, O.; Clément, N. D.; Freixa, Z.; Ruiz, A.; Claver, C.; van Leeuwen, P. W. N. M. Tetrahedron: Asymmetry 2011, 22, 1490.[81] Li, J.; Chen, G.; Wang, Z.; Zhang, R.; Zhang, X.; Ding, K. Chem. Sci. 2011, 2, 1141.[82] Li, J.; Pan, W.; Wang, Z.; Zhang, X.; Ding, K. Adv. Synth. Catal. 2012, 354, 1980.[83] Wu, S.-L.; Zhang, W.-C.; Zhang, Z.-G.; Zhang, X.-M. Org. Lett. 2004, 6, 3565.[84] Zhang, W.; Wang, C.-J.; Gao, W.; Zhang, X. Tetrahedron Lett. 2005, 46, 6087.[85] Hou, X.-H.; Xie, J.-H.; Wang, Q.-S.; Zhou, Q.-L. Adv. Synth. Catal. 2007, 349, 2477.[86] Khlebnikov, A. F.; Kozhushkov, S. I.; Yufit, D. S.; Schill, H.; Reggelin, M.; Spohr, V.; de Meijere, A. Eur. J. Org. Chem. 2012, 1530.[87] Shibatomi, K.; Muto, T.; Sumikawa, Y.; Narayama, A.; Iwasa, S. Synlett 2009, 241.[88] (a) Shibatomi, K.; Narayama, A.; Soga, Y.; Muto, T.; Iwasa, S. Org. Lett. 2011, 13, 2944.(b) Shibatomi, K.; Soga, Y.; Narayama, A.; Fujisawa, I.; Iwasa, S. J. Am. Chem. Soc. 2012, 134, 9836.(c) Narayama, A.; Shibatomi, K.; Soga, Y.; Muto, T.; Iwasa, S. Synlett 2013, 375. |
| [1] | 刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣. 高稳定二维联咔唑sp2碳共轭共价有机框架材料用于高效电催化氧还原★[J]. 化学学报, 2023, 81(8): 884-890. |
| [2] | 刘霞, 匡春香, 苏长会. 过渡金属催化的1,2,3-三氮唑导向的C—H键官能团化反应研究进展[J]. 化学学报, 2022, 80(8): 1135-1151. |
| [3] | 曾誉, 吕品, 蔡跃进, 高福杰, 卓欧, 吴强, 杨立军, 王喜章, 胡征. 分级结构碳纳米笼高效催化苄胺氧化偶联制N-苄烯丁胺[J]. 化学学报, 2021, 79(4): 539-544. |
| [4] | 江崇国, 陈斯嘉, 龚建贤, 杨震. Phainanoids的4,5-螺环骨架的合成探索[J]. 化学学报, 2020, 78(9): 928-932. |
| [5] | 张雷, 马海燕. 亚乙基桥联多取代茚-芴锆、铪配合物的合成、结构及催化丙烯选择性齐聚研究[J]. 化学学报, 2020, 78(8): 778-787. |
| [6] | 黄文姣, 张浩宇, 胡硕真, 钮东方, 张新胜. 酞菁钴催化剂载体表面含氮官能团对其在燃料电池中氧还原性能的影响[J]. 化学学报, 2018, 76(9): 723-728. |
| [7] | 钟国玉, 王红娟, 余皓, 彭峰. 氧还原碳基非贵金属电催化剂研究进展[J]. 化学学报, 2017, 75(10): 943-966. |
| [8] | 程清卿, 许唤, 朱守非, 周其林. 铜催化α-重氮酮对硼氢键的不对称插入反应[J]. 化学学报, 2015, 73(4): 326-329. |
| [9] | 刘勇兵, 杜海峰. “受阻路易斯酸碱对”催化的不对称氢化反应[J]. 化学学报, 2014, 72(7): 771-777. |
| [10] | 齐随涛, 黄俊, 陈昊, 高子丰, 伊春海, 杨伯伦. 有机氢化物可逆储氢循环中脱氢催化剂的研究进展[J]. 化学学报, 2012, 70(24): 2467-2474. |
| [11] | 郑龙珍, 陶堃, 熊乐艳, 叶丹, 韩奎, 纪忆. 碱性介质中Fe/N/C催化剂的氧气还原反应催化性能研究[J]. 化学学报, 2012, 70(22): 2342-2346. |
| [12] | 谢建华, 周其林. 金属催化的不对称氢化反应研究进展与展望[J]. 化学学报, 2012, 70(13): 1427-1438. |
| [13] | 王永霞, 段雪梅, 王钦, 李悦生, 刘靖尧. β-二酮亚胺钛化合物催化乙烯和环戊二烯共聚合反应机理的密度泛函理论研究[J]. 化学学报, 2011, 69(18): 2085-2091. |
| [14] | 周蓉, 张红梅, 杜玉扣, 杨平. 电沉积Pt-Au双金属催化剂及其对甲酸的电催化研究[J]. 化学学报, 2011, 69(13): 1533-1539. |
| [15] | 袁建超, 刘玉凤, 梅铜简, 王学虎. 高活性a-二亚胺基Ni(II)配合物的合成、表征及其催化乙烯聚合研究[J]. 化学学报, 2011, 69(07): 798-802. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||