化学学报 ›› 2019, Vol. 77 ›› Issue (9): 922-926.DOI: 10.6023/A19050158 上一篇 下一篇
所属专题: 有机自由基化学
研究论文
汤娜娜a†, 邵鑫a†, 王明扬a, 吴新鑫a, 朱晨ab*( )
)
投稿日期:2019-05-01
发布日期:2019-06-04
通讯作者:
朱晨
E-mail:chzhu@suda.edu.cn
基金资助:
Tang, Nanaa†, Shao, Xina†, Wang, Mingyanga, Wu, Xinxina, Zhu, Chenab*( )
)
Received:2019-05-01
Published:2019-06-04
Contact:
Zhu, Chen
E-mail:chzhu@suda.edu.cn
Supported by:文章分享

利用自由基参与的远端炔基迁移策略实现了非活化烯烃的炔基化反应. 以磺酰氯为试剂, 在可见光促进下形成三氟甲基自由基或芳基(烷基)磺酰基自由基, 随后加成到非活化烯烃上, 生成烷基自由基中间体, 继而诱导远端炔烃的分子内迁移, 得到三氟甲基炔基化或磺酰基炔基化产物. 该方法具有条件温和、底物易得以及官能团兼容性广等优点.
汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨. 磺酰氯参与的基于远端炔基迁移的非活化烯烃炔基化反应[J]. 化学学报, 2019, 77(9): 922-926.
Tang, Nana, Shao, Xin, Wang, Mingyang, Wu, Xinxin, Zhu, Chen. Sulfonyl Chlorides Mediated Alkynylation of Non-activated Alkenes via Distal Alkynyl Group Migration[J]. Acta Chimica Sinica, 2019, 77(9): 922-926.
| Entry | Photocatalyst (3 mol%) | Base (x equiv.) | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir{[dF(CF3)ppy]2-(dtbbpy)}PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 2 | Ir(ppy)2(dtbbpy)PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 3 | Ru(bpy)3Cl2?6H2O | Na2CO3 (1.0) | CH3CN | <5 |
| 4 | fac-Ir(ppy)3 | Na2CO3 (1.0) | CH3CN | 75 |
| 5 | fac-Ir(ppy)3 | Na2CO3 (0.8) | CH3CN | 57 |
| 6 | fac-Ir(ppy)3 | Na2CO3 (1.2) | CH3CN | 68 |
| 7 | fac-Ir(ppy)3 | Na2CO3 (1.0) | acetone | 68 |
| 8 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DCM | 58 |
| 9 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMSO | <10 |
| 10 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMF | <10 |
| Entry | Photocatalyst (3 mol%) | Base (x equiv.) | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir{[dF(CF3)ppy]2-(dtbbpy)}PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 2 | Ir(ppy)2(dtbbpy)PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 3 | Ru(bpy)3Cl2?6H2O | Na2CO3 (1.0) | CH3CN | <5 |
| 4 | fac-Ir(ppy)3 | Na2CO3 (1.0) | CH3CN | 75 |
| 5 | fac-Ir(ppy)3 | Na2CO3 (0.8) | CH3CN | 57 |
| 6 | fac-Ir(ppy)3 | Na2CO3 (1.2) | CH3CN | 68 |
| 7 | fac-Ir(ppy)3 | Na2CO3 (1.0) | acetone | 68 |
| 8 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DCM | 58 |
| 9 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMSO | <10 |
| 10 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMF | <10 |
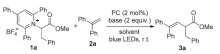
| Entry | CF3SO2Cl (x equiv.) | Photocatalyst (y mol%) | Base (z equiv.) | Solvent | Yield/% |
|---|---|---|---|---|---|
| 1 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | CH3CN | 29 |
| 2 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | acetone | 50 |
| 3 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | MeOH | 45 |
| 4 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMA | 33 |
| 5 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMSO | ND |
| 6 | 1.5 | fac-Ir(ppy)3 (3%) | Na2HPO4 (0.8) | acetone | 40 |
| 7 | 1.5 | fac-Ir(ppy)3 (3%) | Na2CO3 (0.8) | acetone | 36 |
| 8 | 1.5 | fac-Ir(ppy)3 (3%) | K2CO3 (0.8) | acetone | 42 |
| 9 | 1.5 | fac-Ir(ppy)3 (3%) | NaHCO3 (0.8) | acetone | 48 |
| 10 | 1.5 | fac-Ir(ppy)3 (3%) | DABCO (0.8) | acetone | 27 |
| 11 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.7) | acetone | 43 |
| 12 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.9) | acetone | 47 |
| 13 | 1.5 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 52 |
| 14 | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 54 |
| 15b | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 64 |
| 16b | 2.2 | Ru(bpy)3Cl2?6H2O (4%) | K2HPO4 (0.8) | acetone | ND |
| 17b | 2.2 | Ir(ppy)2(dtbbpy)PF6 (4%) | K2HPO4 (0.8) | acetone | ND |
| 18b | 2.2 | none | K2HPO4 (0.8) | acetone | ND |
| 19b,c | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | ND |
| Entry | CF3SO2Cl (x equiv.) | Photocatalyst (y mol%) | Base (z equiv.) | Solvent | Yield/% |
|---|---|---|---|---|---|
| 1 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | CH3CN | 29 |
| 2 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | acetone | 50 |
| 3 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | MeOH | 45 |
| 4 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMA | 33 |
| 5 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMSO | ND |
| 6 | 1.5 | fac-Ir(ppy)3 (3%) | Na2HPO4 (0.8) | acetone | 40 |
| 7 | 1.5 | fac-Ir(ppy)3 (3%) | Na2CO3 (0.8) | acetone | 36 |
| 8 | 1.5 | fac-Ir(ppy)3 (3%) | K2CO3 (0.8) | acetone | 42 |
| 9 | 1.5 | fac-Ir(ppy)3 (3%) | NaHCO3 (0.8) | acetone | 48 |
| 10 | 1.5 | fac-Ir(ppy)3 (3%) | DABCO (0.8) | acetone | 27 |
| 11 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.7) | acetone | 43 |
| 12 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.9) | acetone | 47 |
| 13 | 1.5 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 52 |
| 14 | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 54 |
| 15b | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 64 |
| 16b | 2.2 | Ru(bpy)3Cl2?6H2O (4%) | K2HPO4 (0.8) | acetone | ND |
| 17b | 2.2 | Ir(ppy)2(dtbbpy)PF6 (4%) | K2HPO4 (0.8) | acetone | ND |
| 18b | 2.2 | none | K2HPO4 (0.8) | acetone | ND |
| 19b,c | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | ND |


| [1] |
For selected reviews: (a) Chen, F.; Wang, T.; Jiao, N . Chem. Re. 2014, 114, 8613;
doi: 10.6023/cjoc201803053 |
|
(b) Souillart, L.; Cramer, N . Chem. Re. 2015, 115, 9410;
doi: 10.6023/cjoc201803053 |
|
|
(c) Song, F.; Gou, T.; Wang, B.-Q.; Shi, Z.-J . Chem. Soc. Rev. 2018, 47, 7078;
doi: 10.6023/cjoc201803053 |
|
|
(d) Wu, X.; Zhu, C . Chem. Rec. 2018, 18, 587;
doi: 10.6023/cjoc201803053 |
|
|
(e) Wu, X.; Zhu, C . Chin. J. Chem. 2019, 37, 171;
doi: 10.6023/cjoc201803053 |
|
|
(f) Wang, B.; Liu, Y.; Hao, Z.; Hou, J.; Li, J.; Li, S.; Pan, S.; Zeng, M.; Wang, Z . Chin. J. Org. Chem. 2018, 38, 1872 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳 , 有机化学, 2018, 38, 1872.)
doi: 10.6023/cjoc201803053 |
|
|
(g) Liang, T.; Jiang, L.; Gan, M.; Su, X.; Li, Z . Chin. J. Org. Chem. 2017, 37, 3096 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 梁婷婷, 姜岚, 干苗苗, 苏鑫, 李争宁 , 有机化学, 2017, 37, 3096.)
doi: 10.6023/cjoc201803053 |
|
|
(h) Wu, K.; Song, C.; Cui, D . Chin. J. Org. Chem. 2017, 37, 586 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 吴空, 宋婵, 崔冬梅 , 有机化学, 2017, 37, 586.)
doi: 10.6023/cjoc201803053 |
|
|
(i) Zhong, J.-J.; Meng, Q.-Y.; Chen, B., Tung, C.-H.; Wu, L.-Z . Acta Chim. Sinica. 2017, 75, 34 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 钟建基, 孟庆元, 陈彬, 佟振合, 吴骊珠 , 化学学报, 2017, 75, 34.)
doi: 10.6023/cjoc201803053 |
|
| [2] |
For selected reviews on FGM.(a) Wu, X.; Wu, S.; Zhu, C . Tetrahedron Let. 2018, 59, 1328;
doi: 10.6023/A18080313 |
|
(b) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C . Chem. Soc. Re. 2018, 47, 654;
doi: 10.6023/A18080313 |
|
|
For selected examples from our group, see: (c) Wu, Z.; Ren, R.; Zhu, C.; . Angew. Chem. Int. Ed. 2016, 55, 10821;
doi: 10.6023/A18080313 |
|
|
(d) Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C . J. Am. Chem. Soc. 2017, 139, 1388;
doi: 10.6023/A18080313 |
|
|
(e) Ren, R.; Wu, Z.; Huan, L.; Zhu, C . Adv. Synth. Catal. 2017, 359, 3052;
doi: 10.6023/A18080313 |
|
|
(f) Ji, M.; Wu, Z.; Yu, J.; Wan, X.; Zhu, C . Adv. Synth. Catal. 2017, 359, 1959;
doi: 10.6023/A18080313 |
|
|
(g) Wang, M.; Wu, Z.; Zhang, B.; Zhu, C . Org. Chem. Front. 2018, 5, 1896;
doi: 10.6023/A18080313 |
|
|
(h) Yu, J.; Wang, D.; Xu, Y.; Wu, Z.; Zhu, C . Adv. Synth. Catal. 2018, 360, 744;
doi: 10.6023/A18080313 |
|
|
(i) Zhang, H.; Wu, X.; Zhao, Q.; Zhu, C . Chem. Asian J. 2018, 13, 2453;
doi: 10.6023/A18080313 |
|
|
(j) Chen, D.; Wu, Z.; Yao, Y.; Zhu, C . Org. Chem. Front. 2018, 5, 2370;
doi: 10.6023/A18080313 |
|
|
(k) Tang, N.; Yang, S.; Wu, X.; Zhu, C . Tetrahedron. 2019, 75, 1639;
doi: 10.6023/A18080313 |
|
|
(l) Chen, D.; Ji, M.; Yao, Y.; Zhu, C . Acta Chim. Sinica. 2018, 76, 951 (in Chinese).
doi: 10.6023/A18080313 |
|
|
( 陈栋, 吉梅山, 姚英明, 朱晨 , 化学学报, 2018, 76, 951.)
doi: 10.6023/A18080313 |
|
|
(m) Ji, M.; Wu, Z.; Zhu, C . Chem. Commun. 2019, 55, 2368.
doi: 10.6023/A18080313 |
|
| [3] |
For selected examples of remote FGM from other groups.(a) Thaharn, W.; Soorukram, D.; Kuhakarn, C.; Tuchinda, P.; Reutrakul, V.; Pohmakotr, M . Angew. Chem. Int. E. 2014, 53, 2212;
doi: 10.1002/anie.201310747 |
|
(b) Li, L.; Li, Z.-L.; Wang, F.-L.; Guo, Z.; Cheng, Y.-F.; Wang, N.; Dong, X.-W.; Fang, C.; Liu, J.; Hou, C.; Tan, B.; Liu, X.-Y. . Nat. Commu. 2016, 7, 13852;
doi: 10.1002/anie.201310747 |
|
|
(c) Li, Z.-L.; Li, X.-H.; Wang, N.; Yang, N.-Y.; Liu, X.-Y . Angew. Chem. Int. Ed. 2016, 55, 15100;
doi: 10.1002/anie.201310747 |
|
|
(d) Li, L.; Li, Z.-L.; Gu, Q.-S.; Wang, N.; Liu, X.-Y . Sci. Adv. 2017, 3, e1701487;
doi: 10.1002/anie.201310747 |
|
|
(e) Wang, N.; Li, L.; Li, Z.-L.; Yang, N.-Y.; Guo, Z.; Zhang, H.-X.; Liu, X.-Y . Org. Lett. 2016, 18, 6026;
doi: 10.1002/anie.201310747 |
|
|
(f) Tang, X.; Studer, A . Angew. Chem. Int. Ed. 2018, 57, 814;
doi: 10.1002/anie.201310747 |
|
|
(g) Wang, H.; Xu, Q.; Yu, S . Org. Chem. Front. 2018, 5, 2224;
doi: 10.1002/anie.201310747 |
|
|
(h) He, Y.; Wang, Y.; Gao, J.; Zeng, L.; Li, S.; Wang, W.; Zheng, X.; Zhang, S.; Gu, L.; Li, G . Chem. Commun. 2018, 54, 7499;
doi: 10.1002/anie.201310747 |
|
|
(i) Gu, L.; Gao, Y.; Ai, X.; Jin, C.; He, Y.; Li, G.; Yuan, M . Chem. Commun. 2017, 53, 12946;
doi: 10.1002/anie.201310747 |
|
|
(j) Wei, X.-J.; Noël, T . J. Org. Chem. 2018, 83, 11377.
doi: 10.1002/anie.201310747 |
|
| [4] |
Xu, Y.; Wu, Z.; Jiang, J.; Ke, Z.; Zhu, C . Angew. Chem. Int. E. 2017, 56, 4545.
doi: 10.1002/anie.201700413 |
| [5] | For selected examples of alkynyl migration.(a) Tang, X.; Studer, A . Chem. Sc. 2017, 8, 6888 |
| (b) Liu, J.; Li, W.; Xie, J.; Zhu, C . Org. Chem. Fron. 2018, 5, 797; | |
| (c) Zhao, Q.; Ji, X.-S.; Gao, Y.-Y.; Hao, W.-J.; Zhang, K.-Y.; Tu, S.-J.; Jiang, B . Org. Lett. 2018, 2. 3596. | |
| [6] |
(a) Meadows, D. C.; Gervay-Hague, J . Med. Res. Re. 2006, 26, 793;
doi: 10.1039/C6OB02424F |
|
(b) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X . Curr. Top. Med. Che. 2016, 16, 1200;
doi: 10.1039/C6OB02424F |
|
|
(c) Shaaban, S.; Liang, S.; Liu, N.-W.; Manolikakes, G . Org. Biomol. Chem. 2017, 15, 1947;
doi: 10.1039/C6OB02424F |
|
|
(d) Qiu, G.; Zhou, K.; Gao, L.; Wu, J . Org. Chem. Front. 2018, 5, 691.
doi: 10.1039/C6OB02424F |
| [1] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [2] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [3] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [4] | 杨民, 叶柏柏, 陈健强, 吴劼. 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022, 80(1): 11-15. |
| [5] | 陈栋, 吉梅山, 姚英明, 朱晨. 通过远端碳氮双键迁移实现非活化烯烃的三氟甲硫基化反应[J]. 化学学报, 2018, 76(12): 951-955. |
| [6] | 李曼, 康会英, 薛小松, 程津培. 常见三氟甲基源释放三氟甲基自由基能力的理论计算研究[J]. 化学学报, 2018, 76(12): 988-996. |
| [7] | 苟宝权, 杨超, 张磊, 夏吾炯. 可见光促进的双键三氟甲基化反应研究[J]. 化学学报, 2017, 75(1): 66-69. |
| [8] | 荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波. 可见光促进下氟烷基砜对芳基烯烃的自由基氟烷基化反应[J]. 化学学报, 2017, 75(1): 105-109. |
| [9] | 范雪峰, 赵会君, 朱晨. 羰基兼容的氟代反应及远端氟代脂肪酮合成的近期进展[J]. 化学学报, 2015, 73(10): 979-983. |
| [10] | 朱海涛, 刘国生. 钯催化苯乙烯类分子胺氟化反应研究[J]. 化学学报, 2012, 70(23): 2404-2407. |
| [11] | 潘菲, 施章杰. 过渡金属催化碳氢键三氟甲基化[J]. 化学学报, 2012, 70(16): 1679-1681. |
| [12] | 韩梅,施栩翎,杨镇军,蔡孟深. 1,2,4-三嗪类化合物的研究XXI.3-甲硫基-5-氧代-2.5-二氧-1,2,4-三嗪的取代苯磺铣化反应及6- 碳的亲电特性[J]. 化学学报, 1994, 52(4): 412-416. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||