化学学报 ›› 2022, Vol. 80 ›› Issue (8): 1066-1070.DOI: 10.6023/A22020078 上一篇 下一篇
研究论文
投稿日期:2022-02-20
发布日期:2022-09-01
通讯作者:
刘思敏
基金资助:
Shimin Zhu, Xin Huang, Xie Han, Simin Liu( )
)
Received:2022-02-20
Published:2022-09-01
Contact:
Simin Liu
Supported by:文章分享
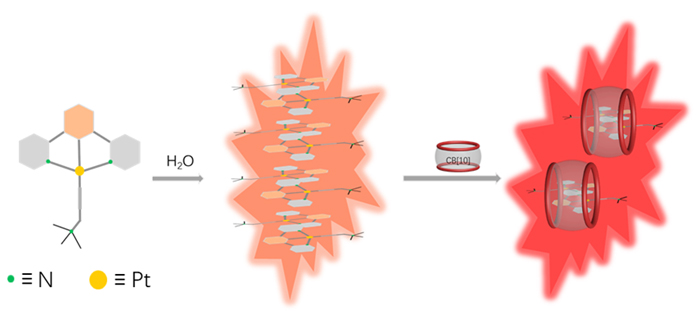
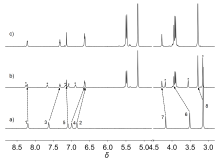
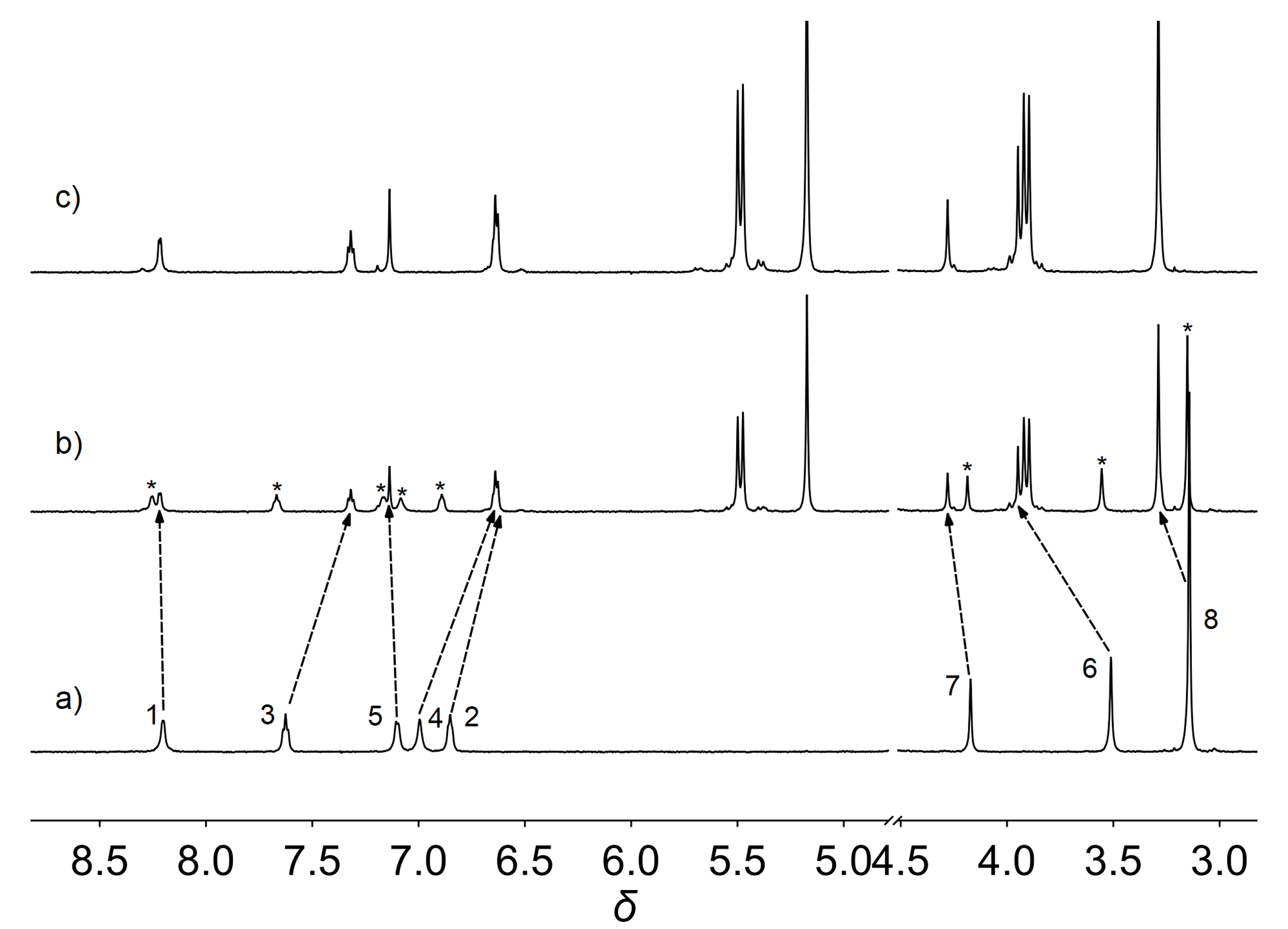
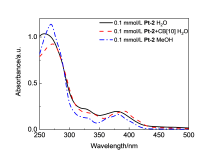
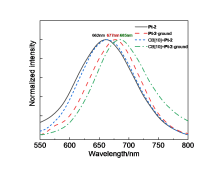
由于过渡金属配合物具有独特的光物理化学性质而被广泛研究. 其中Pt(Ⅱ)配合物发生组装时会因Pt(Ⅱ)-Pt(Ⅱ)之间的距离不同而显示不同的荧光特性, 而主客体相互作用可以影响发光小分子的排列及组装. 为进一步探究主客体相互作用对Pt(Ⅱ)配合物发光性能的影响, 设计合成了不同取代的N^C^N型Pt(Ⅱ)配合物, 研究了大环主体葫芦[10]脲(CB[10])对这类配合物的识别作用及包合物的光谱性质. 核磁共振氢谱和质谱证明CB[10]可与配合物以1∶2的比例结合. 紫外-可见吸收光谱和荧光发射光谱分析表明主客体作用对该类金属配合物光谱性质有较大影响, 所形成的主客体包合物的磷光寿命及量子产率都有不同程度的变化. 研究结果表明, CB[10]可通过包结两个Pt(Ⅱ)配合物分子, 拉近铂原子之间的距离, 增强该类配合物在水相中的Pt(II)…Pt(II)相互作用和π-π相互作用, 实现水相中的长寿命磷光发射. 同时, 主客体作用对这类金属配合物的力致变色性质也有一定的影响.
朱诗敏, 黄鑫, 韩勰, 刘思敏. N^C^N型Pt(II)配合物与大环主体葫芦[10]脲的识别及发光性质研究[J]. 化学学报, 2022, 80(8): 1066-1070.
Shimin Zhu, Xin Huang, Xie Han, Simin Liu. Recognition and Luminescence Properties of N^C^N Pt(II) Complexes with Macrocyclic Host Cucurbit[10]uril[J]. Acta Chimica Sinica, 2022, 80(8): 1066-1070.
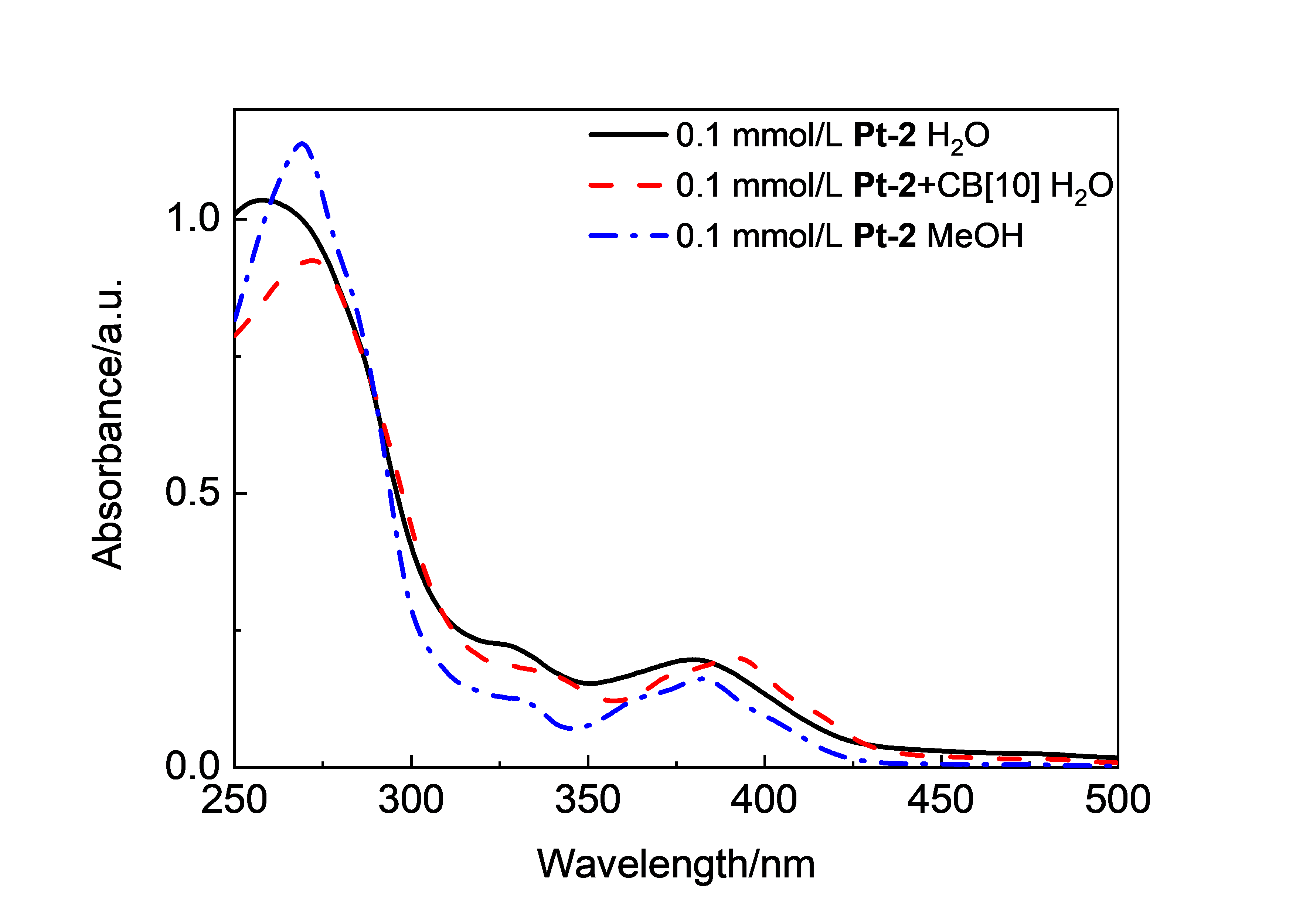
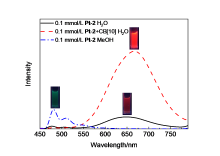

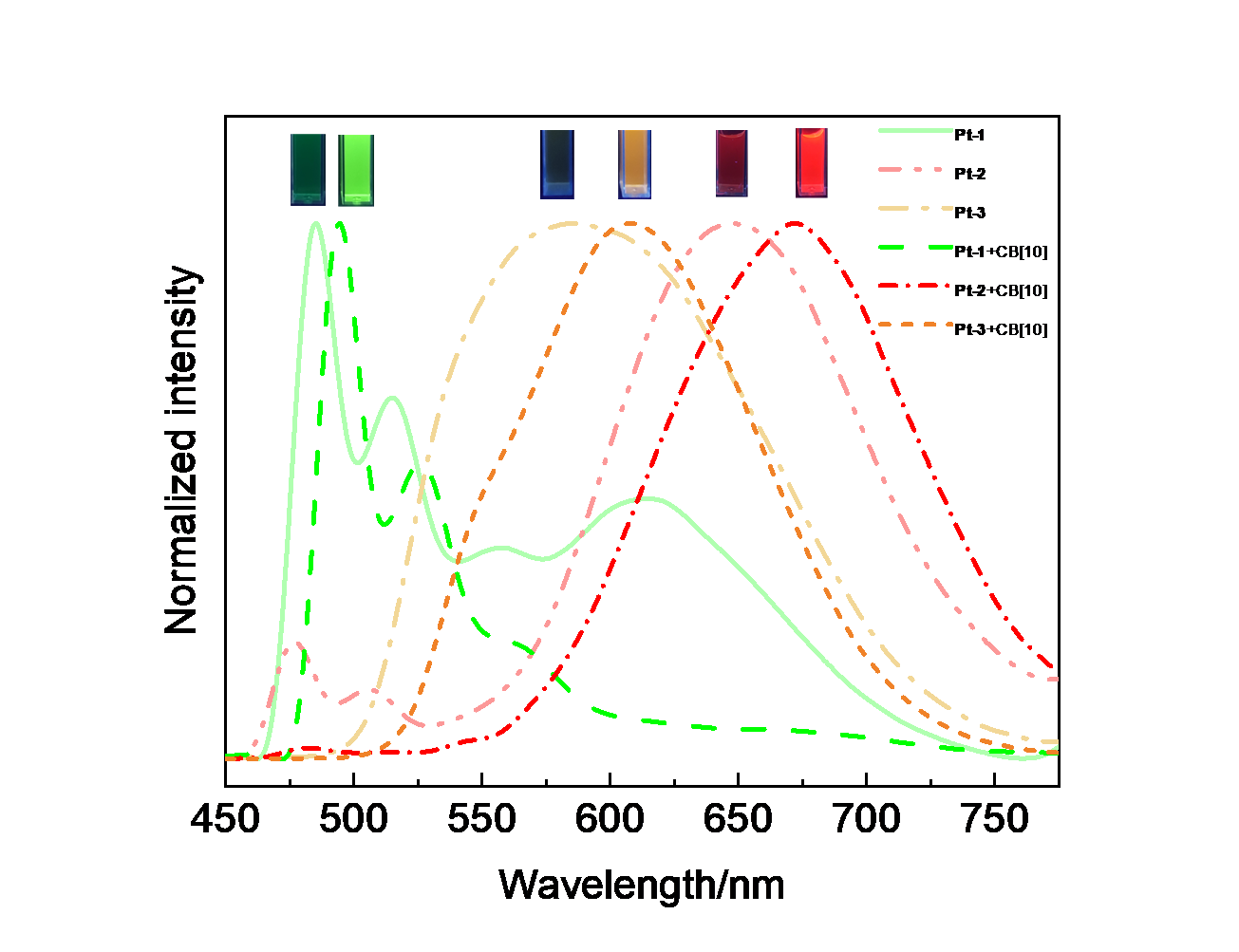
| Complex | λmaxa/nm | τ0/μs | φem/% |
|---|---|---|---|
| Pt-1 | 485 | 13.01 | 2.2 |
| Pt-1+CB[10] | 495 | 20.89 | 16.6 |
| Pt-2 | 648 | 14.64 | 2.7 |
| Pt-2+CB[10] | 667 | 23.74 | 42.0 |
| Pt-3 | 585 | 19.78 | 1.9 |
| Pt-3+CB[10] | 608 | 22.59 | 5.3 |
| Complex | λmaxa/nm | τ0/μs | φem/% |
|---|---|---|---|
| Pt-1 | 485 | 13.01 | 2.2 |
| Pt-1+CB[10] | 495 | 20.89 | 16.6 |
| Pt-2 | 648 | 14.64 | 2.7 |
| Pt-2+CB[10] | 667 | 23.74 | 42.0 |
| Pt-3 | 585 | 19.78 | 1.9 |
| Pt-3+CB[10] | 608 | 22.59 | 5.3 |

| [1] |
(a) Wong, K. M.-C.; Yam, V. W.-W. Acc. Chem. Res. 2011, 44, 424.
doi: 10.1021/ar100130j |
|
(b) Lai, P.-N.; Brysacz, C. H.; Alam, M. K.; Ayoub, N. A.; Gray, T. G.; Bao, J.; Teets, T. S. J. Am. Chem. Soc. 2018, 140, 10198.
doi: 10.1021/jacs.8b04841 |
|
| [2] |
(a) Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N. S.; De Cola, L. Chem. Soc. Rev. 2014, 43, 4144.
doi: 10.1039/C3CS60453E pmid: 25359275 |
|
(b) Chan, A. K.-W.; Ng, M.; Wong, Y.-C.; Chan, M.-Y.; Wong, W.-T.; Yam, V. W.-W. J. Am. Chem. Soc. 2017, 139, 10750.
doi: 10.1021/jacs.7b04952 pmid: 25359275 |
|
|
(c) Lin, J.; Zou, C.; Zhang, X.; Gao, Q.; Suo, S.; Zhuo, Q.; Chang, X.; Xie, M.; Lu, W. Dalton Trans. 2019, 48, 10417.
doi: 10.1039/C9DT02525A pmid: 25359275 |
|
|
(d) Allampally, N. K.; Bredol, M.; Strassert, C. A.; De Cola, L. Chem.-Eur. J. 2014, 20, 16863.
doi: 10.1002/chem.201403772 pmid: 25359275 |
|
| [3] |
Li, K.; Ming Tong, G. S.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Chem. Sci. 2016, 7, 1653.
doi: 10.1039/C5SC03766B |
| [4] |
Chow, P.-K.; To, W.-P.; Low, K.-H.; Che, C.-M. Chem.-Asian J. 2014, 9, 534.
doi: 10.1002/asia.201301059 |
| [5] |
Wang, D.; Chen, Y.; Liu, X.; Bian, J.; Yin, X.; Teng, M.; Rong, M.; Wang, Z. Chin. J. Inorg. Chem. 2021, 37, 33. (in Chinese)
|
|
(王登强, 陈宇, 刘小庆, 卞健健, 尹新颖, 滕明瑜, 戎梅竹, 汪正良, 无机化学学报, 2021, 37, 33.)
|
|
| [6] |
Xu, G; Li, J.; Chen, Z. Acta Chim. Sinica 2014, 72, 667. (in Chinese)
doi: 10.6023/A14030217 |
|
(徐广涛, 李佳, 陈忠宁, 化学学报, 2014, 72, 667.)
doi: 10.6023/A14030217 |
|
| [7] |
Zhang, S.; Wang, S.; Zhou, X.; Zhang, H.; Shao, S.; Li, C. Acta Chim. Sinica 2008, 66, 841. (in Chinese)
|
|
(张首才, 王嵩, 周欣, 张红星, 邵琛, 李传碧, 化学学报, 2008, 66, 841.)
|
|
| [8] |
(a) Sun, C.-Y.; To, W.-P.; Hung, F.-F.; Wang, X.-L.; Su, Z.-M.; Che, C.-M. Chem. Sci. 2018, 9, 2357.
doi: 10.1039/C7SC04528J |
|
(b) Li, Z.; Han, Y.; Gao, Z.; Wang, F. ACS Catal. 2017, 7, 4676.
doi: 10.1021/acscatal.7b00709 |
|
| [9] |
(a) Baggaley, E.; Botchway, S. W.; Haycock, J. W.; Morris, H.; Sazanovich, I. V.; Williams, J. A. G.; Weinstein, J. A. Chem. Sci. 2014, 5, 879.
doi: 10.1039/C3SC51875B |
|
(b) Ouyang, C.; Li, Y.; Rees, T. W.; Liao, X.; Jia, J.; Chen, Y.; Zhang, X.; Ji, L.; Chao, H. Angew. Chem., nt. Ed. 2021, 60, 4150.
|
|
|
(c) Law, A. S.-Y.; Lee, L. C.-C.; Lo, K. K.-W.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 5396.
doi: 10.1021/jacs.0c13327 |
|
| [10] |
(a) Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Inorg. Chem. 2021, 60, 10350.
doi: 10.1021/acs.inorgchem.1c00822 |
|
(b) Ramu, V.; Gautam, S.; Garai, A.; Kondaiah, P.; Chakravarty, A. R. Inorg. Chem. 2018, 57, 1717.
doi: 10.1021/acs.inorgchem.7b02249 |
|
| [11] |
(a) Summa, G. M.; Scott, B. A. Inorg. Chem. 1980, 19, 1079.
doi: 10.1021/ic50206a064 |
|
(b) Yip, H.-K.; Cheng, L.-K.; Cheung, K.-K.; Che, C.-M. J. Chem. Soc., Dalton Trans. 1993, 2933.
|
|
| [12] |
(a) Yam, V. W.-W.; Tang, R. P.-L.; Wong, K. M.-C.; Cheung, K.-K. Organometallics 2001, 20, 4476.
doi: 10.1021/om010336x |
|
(b) Du, P.; Schneider, J.; Jarosz, P.; Eisenberg, R. J. Am. Chem. Soc. 2006, 128, 7726.
doi: 10.1021/ja0610683 |
|
|
(c) Yang, Z.; Tian, Y.; Li, Z.; Ao, L.; Gao, Z.; Wang, F. Acta Polym. Sin. 2017, 48, 121. (in Chinese)
|
|
|
(杨支帅, 田玉奎, 李子健, 敖雷, 高宗春, 汪峰, 高分子学报, 2017, 48, 121.)
|
|
| [13] |
(a) Yam, V. W.-W.; Wong, K. M.-C.; Zhu, N. J. Am. Chem. Soc. 2002, 124, 6506.
doi: 10.1021/ja025811c |
|
(b) Yam, V. W.-W.; Chan, K. H.-Y.; Wong, K. M.-C.; Zhu, N. Chem.-Eur. J. 2005, 11, 4535.
doi: 10.1002/chem.200500106 |
|
| [14] |
Williams, J. A. G. Chem. Soc. Rev. 2009, 38, 1783.
doi: 10.1039/b804434c pmid: 19587968 |
| [15] |
(a) Hu, S.-J.; Guo, X.-Q.; Zhou, L.-P.; Yan, D.-N.; Cheng, P.-M.; Cai, L.-X.; Li, X.-Z.; Sun, Q.-F. J. Am. Chem. Soc. 2022, 144, 4244.
doi: 10.1021/jacs.2c00760 |
|
(b) Gemen, J.; Ahrens, J.; Shimon, L. J. W.; Klajn, R. J. Am. Chem. Soc. 2020, 142, 17721.
doi: 10.1021/jacs.0c08589 |
|
|
(c) Benson, C. R.; Kacenauskaite, L.; VanDenburgh, K. L.; Zhao, W.; Qiao, B.; Sadhukhan, T.; Pink, M.; Chen, J.; Borgi, S.; Chen, C.-H.; Davis, B. J.; Simon, Y. C.; Raghavachari, K.; Laursen, B. W.; Flood, A. H. Chem 2020, 6, 1978.
doi: 10.1016/j.chempr.2020.06.029 |
|
| [16] |
(a) Liu, S.; Zavalij, P. Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 127, 16798.
doi: 10.1021/ja056287n |
|
(b) Yang, X.; Liu, F.; Zhao, Z.; Liang, F.; Zhang, H.; Liu, S. Chin. Chem. Lett. 2018, 29, 1560.
doi: 10.1016/j.cclet.2018.01.032 |
|
|
(c) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese)
doi: 10.6023/cjoc202003066 |
|
|
(田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.)
doi: 10.6023/cjoc202003066 |
|
| [17] |
(a) Anis-Ul-Haque, K. M.; Woodward, C. E.; Day, A. I.; Wallace, L. Inorg. Chem. 2020, 59, 3942.
doi: 10.1021/acs.inorgchem.9b03603 pmid: 32578987 |
|
(b) Luis, E. T.; Day, A. I.; König, B.; Beves, J. E. Inorg. Chem. 2020, 59, 9135.
doi: 10.1021/acs.inorgchem.0c00986 pmid: 32578987 |
|
|
(c) Zhang, Y.; Liu, M.; Karatchevtseva, I.; Price, J. R.; Tao, Z.; Wei, G. New J. Chem. 2020, 44, 18208.
doi: 10.1039/D0NJ03962D pmid: 32578987 |
|
| [18] |
(a) Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Chem. Commun. 2018, 54, 2169.
doi: 10.1039/C8CC00593A |
|
(b) Deng, Y.; Yin, H.; Zhao, Z.; Wang, R.; Liu, S. Supramol. Chem. 2018, 30, 706.
doi: 10.1080/10610278.2018.1455977 |
|
| [19] |
Hu, Z.; Sun, D.; Han, X.; Liu, S. Chin. J. Org. Chem. 2020, 40, 1361. (in Chinese)
doi: 10.6023/cjoc201912014 |
|
(胡智雄, 孙冬冬, 韩勰, 刘思敏, 有机化学, 2020, 40, 1361.)
doi: 10.6023/cjoc201912014 |
|
| [20] |
(a) Chen, Y.; Li, K.; Lu, W.; Chui, S. S.-Y.; Ma, C.-W.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 9909.
doi: 10.1002/anie.200905678 pmid: 14686833 |
|
(b) Williams, J. A. G.; Beeby, A.; Davies, E. S.; Weinstein, J. A.; Wilson, C. Inorg. Chem. 2003, 42, 8609.
pmid: 14686833 |
|
|
(c) Cárdenas, D. J.; Echavarren, A. M.; Ramírez de Arellano, M. C. Organometallics 1999, 18, 3337.
doi: 10.1021/om990125g pmid: 14686833 |
|
|
(d) Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Inorg. Chem. 2010, 49, 11276.
doi: 10.1021/ic100740e pmid: 14686833 |
|
| [21] |
Wan, Q.; Xiao, X.-S.; To, W.-P.; Lu, W.; Chen, Y.; Low, K.-H.; Che, C.-M. Angew. Chem., nt. Ed. 2018, 57, 17189.
|
| [22] |
(a) Chen, Y.; Lu, W.; Che, C.-M. Organometallics 2013, 32, 350.
doi: 10.1021/om300965b |
|
(b) Ai, Y.; Chan, M. H.-Y.; Chan, A. K.-W.; Ng, M.; Li, Y.; Yam, V. W.-W. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 13856.
doi: 10.1073/pnas.1908034116 |
|
| [23] |
Li, B.; Li, Y.; Chan, M. H.-Y.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 21676.
doi: 10.1021/jacs.1c10943 |
| [24] |
Xie, M.; Lu, W. Dalton Trans. 2019, 48, 1275.
doi: 10.1039/c8dt03707h pmid: 30608080 |
| [25] |
Zhu, S.; Hu, J.; Zhai, S.; Wang, Y.; Xu, Z.; Liu, R.; Zhu, H. Inorg. Chem. Front. 2020, 7, 4677.
doi: 10.1039/D0QI00735H |
| [26] |
Han, X.; Sun, D.; Tang, S.; Wu, Y.; Wang, L.; Zhang, X.; Liu, S. J. Mater. Chem. C 2021, 9, 17307.
doi: 10.1039/D1TC04433H |
| [27] |
(a) Kozhevnikov, V. N.; Donnio, B.; Bruce, D. W. Angew. Chem., nt. Ed. 2008, 47, 6286.
|
|
(b) Choi, S. J.; Kuwabara, J.; Nishimura, Y.; Arai, T.; Kanbara, T. Chem. Lett. 2011, 41, 65.
doi: 10.1246/cl.2012.65 |
|
|
(c) Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Dalton Trans. 2009, 711.
|
|
|
(d) Zhang, X.-P.; Mei, J.-F.; Lai, J.-C.; Li, C.-H.; You, X.-Z. J. Mater. Chem. C 2015, 3, 2350.
doi: 10.1039/C4TC02800G |
| [1] | 汪阳, 阎敬灵. 不同配体的稀土金属配合物在烯烃聚合领域的研究进展[J]. 化学学报, 2023, 81(3): 275-288. |
| [2] | 李波, 周海燕, 马海燕, 黄吉玲. 亚乙基桥联双茚锆、铪配合物的合成及催化丙烯选择性齐聚研究: 茚环3-位取代基的影响[J]. 化学学报, 2023, 81(10): 1280-1294. |
| [3] | 马雪璐, 李蒙, 雷鸣. 三核过渡金属配合物在催化反应中的研究进展[J]. 化学学报, 2023, 81(1): 84-99. |
| [4] | 焦阳, 张希. 超分子自由基:构筑、调控与功能[J]. 化学学报, 2018, 76(9): 659-665. |
| [5] | 崔彬彬, 唐健洪, 钟羽武. 基于过渡金属配合物的阻变存储材料[J]. 化学学报, 2016, 74(9): 726-733. |
| [6] | 李惠梅, 王洁, 倪云洲, 周永丰, 颜德岳. “线性-超支化”超分子聚合物的制备及光响应性自组装行为研究[J]. 化学学报, 2016, 74(5): 415-421. |
| [7] | 张益伟, 马雪璐, 张欣, 雷鸣. 邻苯二硫酚桥联双核双氮过渡金属配合物N-N键活化规律的理论研究[J]. 化学学报, 2016, 74(4): 340-350. |
| [8] | 钱长涛, 王春红, 陈耀峰. 稀土金属有机配合物化学60年[J]. 化学学报, 2014, 72(8): 883-905. |
| [9] | 易君明, 肖欣, 张云黔, 薛赛凤, 陶朱, 张建新. 八元瓜环与1,7-二(2-苯并咪唑)-庚烷的超分子自组装[J]. 化学学报, 2014, 72(8): 949-955. |
| [10] | 孔蕊, 施冬健, 刘蓉瑾, 吴超, 倪沛红, 陈明清. 双敏感性环糊精超分子聚集体的制备及其性能研究[J]. 化学学报, 2013, 71(11): 1540-1546. |
| [11] | 吴祥, 李明丽, 龚流柱. 金属配合物/手性磷酸参与的不对称接力催化反应[J]. 化学学报, 2013, 71(08): 1091-1100. |
| [12] | 张小军, 刘尚钟, 吴学民, 李姝静. 基于环糊精二聚体与紫精聚合物的包结作用制备超分子水凝胶[J]. 化学学报, 2012, 70(19): 2066-2072. |
| [13] | 王新科, Sit Met-Met, 孙杰, 唐勇, 谢作伟. 边臂修饰的水杨醛亚胺第四族金属配合物的合成、结构及其乙烯聚合行为研究[J]. 化学学报, 2012, 70(18): 1909-1916. |
| [14] | 仇毅翔, 王曙光. 三(五甲基环戊二烯基)稀土金属配合物(C5Me5)3Ln (Ln=Sc, Y, La)的量子化学理论研究[J]. 化学学报, 2012, 70(18): 1930-1938. |
| [15] | 李姝静, 张小军, 梁海燕, 王心蕊. 基于环糊精二聚体与金刚烷修饰的温敏性聚合物的主客体识别构筑超分子水凝胶[J]. 化学学报, 2012, 70(08): 1013-1020. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||