化学学报 ›› 2022, Vol. 80 ›› Issue (9): 1223-1230.DOI: 10.6023/A22040169 上一篇 下一篇
研究论文
赵杰a, 王治文a, 李华清b, 艾琦a,*( ), 蔡培庆a, 司俊杰a, 姚鑫a, 胡晓光b,*(
), 蔡培庆a, 司俊杰a, 姚鑫a, 胡晓光b,*( ), 刘祖刚a,*(
), 刘祖刚a,*( )
)
投稿日期:2022-08-03
发布日期:2022-08-09
通讯作者:
艾琦, 胡晓光, 刘祖刚
Jie Zhaoa, Zhiwen Wanga, Huaqing Lib, Qi Aia( ), Peiqing Caia, Junjie Sia, Xin Yaoa, Xiaoguang Hub(
), Peiqing Caia, Junjie Sia, Xin Yaoa, Xiaoguang Hub( ), Zugang Liua(
), Zugang Liua( )
)
Received:2022-08-03
Published:2022-08-09
Contact:
Qi Ai, Xiaoguang Hu, Zugang Liu
文章分享

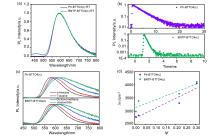
可见光驱动光环化的荧光turn-on型二芳基乙烯分子开关是超分辨显微成像的理想材料, 但目前关于该类型分子的报道仍较少. 本工作合成了一种基于苯并[b]萘并[1,2-d]噻吩(BNTP)的405 nm可见光驱动光环化的荧光turn-on型二芳基乙烯分子BNTP-BTTO4, 同时系统研究了该分子的光物理性能与稳定性, 并借助密度泛函理论(DFT)计算厘清了分子实现可见光驱动光环化及荧光turn-on原因. 另外, 本研究发现BNTP引入后分子表现出比参比分子Ph-BTTO4更优异的抗疲劳性、热稳定性和光稳定性, 尤其是在历经200 min 405 nm可见光照射后, BNTP-BTTO4(c)吸收强度只下降4%, 光稳定性大幅提升. 本研究为设计开发性能优异的可见光驱动光环化的荧光turn-on型二芳基乙烯提供了新的思路.
赵杰, 王治文, 李华清, 艾琦, 蔡培庆, 司俊杰, 姚鑫, 胡晓光, 刘祖刚. 基于苯并[b]萘并[1,2-d]噻吩的可见光驱动光环化荧光turn-on型二芳基乙烯的合成与性能研究[J]. 化学学报, 2022, 80(9): 1223-1230.
Jie Zhao, Zhiwen Wang, Huaqing Li, Qi Ai, Peiqing Cai, Junjie Si, Xin Yao, Xiaoguang Hu, Zugang Liu. Synthesis and Properties of Visible-light-driven Fluorescence Turn-on Diarylethenes Based on Benzo[b]naphtho[1,2-d]thiophene[J]. Acta Chimica Sinica, 2022, 80(9): 1223-1230.



| Ph-BTTO4 | BNTP-BTTO4 | ||||
|---|---|---|---|---|---|
| Open | Closed | Open | Closed | ||
| Theoretical data | |||||
| EVA(S1)a/eV | 3.42 | 2.48 | 3.21 | 2.52 | |
| EHOMOb/eV | 6.20 | 5.50 | 5.92 | 5.54 | |
| ELUMOb/eV | 2.29 | 2.78 | 2.30 | 2.76 | |
| Egap/eV | 3.91 | 2.72 | 3.62 | 2.78 | |
| Experimental data | |||||
| ε/(L•mmol–1•cm–1) | 5.3 | 15.8 | |||
| $\lambda_{max}^{EM}$/nm | 592 | 593 | |||
| ϕF | 0.26 | 0.05 | |||
| τ/ns | 3.22 | 0.19 | |||
| Ph-BTTO4 | BNTP-BTTO4 | ||||
|---|---|---|---|---|---|
| Open | Closed | Open | Closed | ||
| Theoretical data | |||||
| EVA(S1)a/eV | 3.42 | 2.48 | 3.21 | 2.52 | |
| EHOMOb/eV | 6.20 | 5.50 | 5.92 | 5.54 | |
| ELUMOb/eV | 2.29 | 2.78 | 2.30 | 2.76 | |
| Egap/eV | 3.91 | 2.72 | 3.62 | 2.78 | |
| Experimental data | |||||
| ε/(L•mmol–1•cm–1) | 5.3 | 15.8 | |||
| $\lambda_{max}^{EM}$/nm | 592 | 593 | |||
| ϕF | 0.26 | 0.05 | |||
| τ/ns | 3.22 | 0.19 | |||


| [1] |
Jeong Y. C.; Yang S. I.; Ahn K. H.; Kim E. Chem. Commun. 2005, 19, 2503.
|
| [2] |
Jeong Y. C.; Yang S. I.; Kim E.; Ahn K. H. Tetrahedron 2006, 62, 5855.
doi: 10.1016/j.tet.2006.04.029 |
| [3] |
Jeong Y. C.; Han J. P.; Kim Y.; Kim E.; Yang S. I.; Ahn K. H. Tetrahedron 2007, 63, 3173.
doi: 10.1016/j.tet.2007.02.007 |
| [4] |
Uno K.; Niikura H.; Morimoto M.; Ishibashi Y.; Miyasaka H.; Irie M. J. Am. Chem. Soc. 2011, 133, 13558.
doi: 10.1021/ja204583e |
| [5] |
Pang S. C.; Hyun H.; Lee S.; Jang D.; Lee M. J.; Kang S. H.; Ahn K. H. Chem. Commun. 2012, 48, 3745.
doi: 10.1039/c2cc30738c |
| [6] |
Roubinet B.; Bossi M. L.; Alt P.; Leutenegger M.; Shojaei H.; Schnorrenberg S.; Nizamov S.; Irie M.; Belov V. N.; Hell S. W. Angew. Chem., Int. Ed. 2016, 55, 15429.
doi: 10.1002/anie.201607940 pmid: 27767250 |
| [7] |
Yang H.; Li M. Q.; Li C.; Luo Q. F.; Zhu M. Q.; Tian H. Angew. Chem., Int. Ed. 2020, 59, 8560.
doi: 10.1002/anie.201909830 |
| [8] |
Li C.; Xiong K.; Chen Y.; Fan C.; Wang Y. L.; Ye H.; Zhu M. Q. ACS Appl. Mater. Inter. 2020, 12, 27651.
doi: 10.1021/acsami.0c03122 |
| [9] |
Roubinet B.; Weber M.; Shojaei H.; Bates M.; Bossi M. L.; Belov V. N.; Irie M.; Hell S. W. J. Am. Chem. Soc. 2017, 139, 6611.
doi: 10.1021/jacs.7b00274 pmid: 28437075 |
| [10] |
Uno K.; Bossi M. L.; Konen T.; Belov V. N.; Irie M.; Hell S. W. Adv. Opt. Mater. 2019, 7, 1801746.
|
| [11] |
Qiang Z.; Shebek K. M.; Irie M.; Wang M. ACS Macro. Lett. 2018, 7, 1432.
doi: 10.1021/acsmacrolett.8b00686 |
| [12] |
Irie M. Chem. Rev. 2000, 100, 1685.
doi: 10.1021/cr980069d |
| [13] |
Irie M.; Fukaminato T.; Matsuda K.; Kobatake S. Chem. Rev. 2014, 114, 12174.
doi: 10.1021/cr500249p |
| [14] |
Zhang J. J.; Zou Q.; Tian H. Adv. Mater. 2013, 25, 378.
doi: 10.1002/adma.201201521 |
| [15] |
Zhu S.; Li W.; Zhu W. Prog. Chem. 2016, 28, 975.
|
| [16] |
Xu C.; Zhang J.; Xu W.; Tian H. Mater. Chem. Front. 2021, 5, 1060.
doi: 10.1039/D0QM00567C |
| [17] |
Singh R.; Xiao C.-C.; Wei C.-L.; Ho F.-C.; Khang T. M.; Gouda C.; Wu T.-K.; Li Y.-K.; Wei K.-H.; Lin H.-C. Mater. Chem. Front. 2021, 5, 438.
doi: 10.1039/D0QM00605J |
| [44] |
Maegawa R.; Kitagawa D.; Hamatani S.; Kobatake S. New J. Chem. 2021, 45, 18969.
doi: 10.1039/D1NJ04047B |
| [45] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Keith T.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C., Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, Gaussian Inc., Wallingford CT, 2009.
|
| [18] |
Herder M.; Schmidt B. M.; Grubert L.; PätzeL M.; Schwarz J.; Hecht S. J. Am. Chem. Soc. 2015, 137, 2738.
doi: 10.1021/ja513027s |
| [19] |
Yin J.; Kwon Y.; Kim D.; Lee D.; Kim G.; Hu Y.; Ryu J. H.; Yoon J. J. Am. Chem. Soc. 2014, 136, 5351.
doi: 10.1021/ja412628z |
| [20] |
Carling C. J.; Boyer, J. C.; Branda, N. R. J. Am. Chem. Soc. 2009, 131, 10838.
doi: 10.1021/ja904746s pmid: 19722663 |
| [21] |
Boyer J. C.; Carling C. J.; Gates B. D.; Branda N. R. J. Am. Chem. Soc. 2010, 132, 15766.
doi: 10.1021/ja107184z |
| [22] |
Kashihara R.; Morimoto M.; Ito S.; Miyasaka H.; Irie M. J. Am. Chem. Soc. 2017, 139, 16498
doi: 10.1021/jacs.7b10697 pmid: 29112401 |
| [23] |
Mori K.; Ishibashi Y.; Matsuda H.; Ito S.; Nagasawa Y.; Nakagawa H.; Uchida K.; Yokojima S.; Nakamura S.; Irie M.; Miyasaka H. J. Am. Chem. Soc. 2011, 133, 2621.
doi: 10.1021/ja108992t |
| [24] |
Zhang Z. W.; Zhang J. J.; Wu B.; Li X.; Chen Y.; Huang J. H.; Zhu L. L.; Tian H. Adv. Opt. Mater. 2018, 6, 1700847.
|
| [25] |
Zhang Z. W.; Wang W. H.; Jin P. P.; Xue J. D.; Sun L.; Huang J. H.; Zhang J. J.; Tian H. Nat. Commun. 2019, 10, 1.
doi: 10.1038/s41467-018-07882-8 |
| [26] |
Yam V. W. W.; Ko C. C.; Zhu N. Y. J. Am. Chem. Soc. 2004, 126, 12734.
doi: 10.1021/ja047446q |
| [27] |
Poon C. T.; Lam W. H.; Wong H. L.; Yam V. W. W. J. Am. Chem. Soc. 2010, 132, 13992.
doi: 10.1021/ja105537j |
| [28] |
Poon C. T.; Lam W. H.; Yam V. W. W. Chem. Eur. J. 2013, 19, 3467.
doi: 10.1002/chem.201203105 |
| [29] |
Iwai R.; Morimoto M.; Irie M. Photochem. Photobiol. Sci. 2020, 19, 783.
doi: 10.1039/D0PP00064G |
| [30] |
Chen S. J.; Li W. L.; Li X.; Zhu W. H. J. Mater. Chem. C 2017, 5, 2717.
doi: 10.1039/C7TC00023E |
| [31] |
Li Z. Y.; He C. J.; Lu Z. Q.; Li P. S.; Zhu Y. P. Dyes Pigm. 2020, 182, 108623.
doi: 10.1016/j.dyepig.2020.108623 |
| [32] |
Ai Q.; Hong S. J.; Khan M. A.; Ahn K. H. Bull. Korean Chem. Soc. 2018, 39, 1308.
doi: 10.1002/bkcs.11597 |
| [33] |
Wang L. P.; Wang L. M.; Zhang L. Mater. Chem. Phys. 2018, 212, 155.
doi: 10.1016/j.matchemphys.2018.03.026 |
| [34] |
Zhang Q. S.; Kuwabara H.; Potscavage W. J.; Huang S. P.; Hatae Y.; Shibata T.; Adachi C. J. Am. Chem. Soc. 2014, 136, 18070.
doi: 10.1021/ja510144h |
| [35] |
Lakowicz J. R. Principles of Fluorescence Spectroscopy, Springer, New York, 2006, p. 208.
|
| [36] |
Ai Q.; Pang S. C.; Ahn K. H. Chem. Eur. J. 2016, 22, 656.
doi: 10.1002/chem.201504131 |
| [37] |
Wu Y.; Chen S. J.; Yang Y. H.; Zhang Q.; Xie Y. S.; Tian H.; Zhu W. H. Chem. Commun. 2012, 48, 528.
doi: 10.1039/C1CC15824D |
| [38] |
Zhang D. D.; Song X. Z.; Cai M. H.; Kaji H.; Duan L. Adv. Mater. 2018, 30, 1705406.
|
| [39] |
Jia X.; Shao C.; Bai X.; Zhou Q.; Wu B.; Wang L.; Yue B.; Zhu H.; Zhu L. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 4816.
doi: 10.1073/pnas.1821991116 |
| [40] |
Wu H.; Chi W.; Baryshinkov G.; Wu B.; Gong Y.; Zheng D.; Li X.; Zhao Y.; Liu X.; Agren H.; Zhu L. Angew. Chem., Int. Ed. 2019, 58, 4328.
doi: 10.1002/anie.201900703 |
| [41] |
Jia X.; Yue B.; Zhou L.; Niu X.; Wu W.; Zhu L. Chem. Commun. 2020, 56, 4336.
doi: 10.1039/D0CC00371A |
| [42] |
Liu J. Y.; Zhou K. R.; Wang D.; Deng C.; Duan K.; Ai Q.; Zhang Q. S. Front. Chem. 2019, 7, 312.
doi: 10.3389/fchem.2019.00312 |
| [43] |
Kitagawa D.; Nakahama T.; Nakai Y.; Kobatake S. J. Mater. Chem. C 2019, 7, 2865.
doi: 10.1039/C8TC05357J |
| [1] | 刘懿玮, 马良伟, 王巧纯, 马骧. 基于二芳基乙烯的光响应型室温磷光材料★[J]. 化学学报, 2023, 81(5): 445-449. |
| [2] | 拉毛, 包山虎, 莎仁. Pd催化层对含氧氢化钇(YHx:O)薄膜光致变色调节能力的影响[J]. 化学学报, 2019, 77(1): 90-94. |
| [3] | 陈鹏, 王宇洋, 张宇模, 张晓安. 以吖啶酮为母体的双螺吡喃开关分子的设计、合成与性质研究[J]. 化学学报, 2016, 74(8): 669-675. |
| [4] | 王志强, 肖殷, 金会义, 谈廷风, 王世荣, 李祥高. 含三苯胺基团的二噻吩乙烯光致变色化合物的合成及性能研究[J]. 化学学报, 2014, 72(6): 731-738. |
| [5] | 谭春斌, 赵泽琳, 高峻, 雷景新. 新型螺吡喃化合物的合成及应用研究[J]. 化学学报, 2012, 70(9): 1095-1103. |
| [6] | 宋玉民, 张玉梅, 马新贤, 朱早龙, 许军鹏, 刘景旺. 姜黄素稀土大环配合物的光致变色性能研究[J]. 化学学报, 2011, 69(11): 1347-1353. |
| [7] | 庞美丽, 杨涛涛, 李晶晶, 杨素华, 娄志刚, 韩杰, 孟继本. 新型含氮杂环螺吡喃化合物的合成及性能研究[J]. 化学学报, 2010, 68(18): 1895-1902. |
| [8] | 朱华,赵丽,陈美,颜莎宁,张青龙,沈毅. 甲醛诱导合成WO3粉体与光致变色性质研究[J]. 化学学报, 2009, 67(2): 174-178. |
| [9] | 陈勇,庞美丽,程凯歌,王英,韩杰,孟继本. 一种新型的光调控磁分子体系的合成与性能研究[J]. 化学学报, 2008, 66(9): 1091-1096. |
| [10] | 申凯华, 韩建国, 张刚, 崔东熏. 一种支链含有螺吡喃和查尔酮双光功能基团的复合高分子材料光致变色性能研究[J]. 化学学报, 2007, 65(6): 542-546. |
| [11] | 尹京花,连慧琴, 周子彦, 赵继阳, 吴学. 6-羟基-5,12-萘并萘醌及其CH3, C6H5取代衍生物的光致变色理论研究[J]. 化学学报, 2007, 65(24): 2821-2826. |
| [12] | 艾丽梅, 陈捷, 李永仙, 冯威, 刘延, 熊德骐. 多酸含量对无机/高分子复合薄膜结构及光致变色性能的影响[J]. 化学学报, 2007, 65(17): 1841-1844. |
| [13] | 张其震, 殷晓颖, 李爱香. 周边含己氧基的三代光致变色液晶树枝状大分子的光化学[J]. 化学学报, 2006, 64(16): 1743-1748. |
| [14] | 王艳,张其震. 含硝基二代光致变色液晶树枝状大分子的光化学研究[J]. 化学学报, 2006, 64(15): 1593-1600. |
| [15] | 连慧琴,周子彦,侯军,吴学. 苯氧基萘并萘醌衍生物的合成与光致变色性能研究[J]. 化学学报, 2006, 64(10): 1036-1042. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||