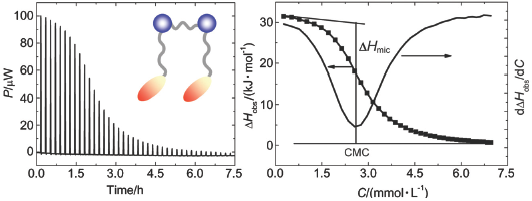
| [1] Rosen, M. J. In Surfactants and Interfacial Phenomena, 3rd ed., John Wiley and Sons, New York, 2004, pp. 105~175. [2] Bashford, M. T.; Woolley, E. M. J. Phys. Chem. 1985, 89, 3173. [3] Woolley, E. M.; Bashford, M. T. 1986, 90, 3038. [4] Kresheck, G. C. J. Phys. Chem. B 1998, 102, 6596. [5] Kresheck, G. C. J. Am. Chem. Soc. 1998, 120, 10964. [6] Li, Y.; Reeve, J.; Wang, Y.; Thomas, R. K.; Wang, J.; Yan, H. J. Phys. Chem. B 2005, 109, 16070. [7] Bai, G.; Yan, H.; Thomas, R. K. Langmuir 2001, 17, 4501. [8] Bai, G.; Wang, J.; Yan, H.; Li, Z.; Thomas, R. K. J. Phys. Chem. B 2001, 105, 3105. [9] Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R. K. J. Phys. Chem. B 2004, 108, 15385. [10] Li, Y.; Li, P.; Dong, C.; Wang, X.; Wang, Y.; Yan, H.; Thomas, R. K. Langmuir 2006, 22, 42. [11] Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed., John Wiley and Sons, New York, 1980. [12] Shinoda, K. J. Phys. Chem. 1977, 81, 1300. [13] Gill, S. J.; Wadso, I. Proc. Natl. Acad. Sci. U. S. A. 1976, 73, 2955. [14] Menger, F. M.; Keiper, J. S. Angew. Chem. Int. Ed. 2000, 39, 1906. [15] Zana, R.; Xia, J. Gemini Surfactants: Synthesis, Interfacial and Solution-Phase Behavior, and Applications, Marcel Dekker, Inc., New York, 2004. [16] Han, Y.; Wang, Y. Phys. Chem. Chem. Phys. 2011, 13, 1939. [17] Steel, W. H.; Damkaci, F.; Nolan, R.; Walker, R. A. J. Am. Chem. Soc. 2002, 124, 4824. [18] Beildeck, C. L.; Steel, W. H.; Walker, R. A. Langmuir 2003, 19, 4933. [19] Steel, W. H.; Walker, R. A. Nature 2003, 424, 296. [20] Huang, X.; Han, Y.; Wang, Y.; Wang, Y. J. Phys. Chem. B 2007, 111, 12439. [21] Huang, X.; Wang, Y.; Dong, C.; Shen, H.-H.; Thomas, R. K. J. Colloid Interface Sci. 2008, 325, 114. [22] Zana, R. Langmuir 1996, 12, 1208. [23] Wang, X.; Wang, J.; Wang, Y.; Ye, J.; Yan, H.; Thomas, R. K. J. Phys. Chem. B 2003, 107, 11428. [24] Zana, R. J. Colloid Interface Sci. 1980, 78, 330. [25] Zana, R.; Benrraou, M.; Rueff, R. Langmuir 1991, 7, 1072. [26] Zana, R.; Lévy, H. Colloids Surf. A: Physicochem. Eng. Aspects 1997, 127, 229. [27] Li, Y.; Li, P.; Wang, J.; Wang, Y.; Yan, H.; Thomas, R. K. Langmuir 2005, 21, 6703. [28] Paula, S.; Sues, W.; Tuchtenhagen, J.; Blume, A. J. Phys. Chem. 1995, 99, 11742. [29] Beyer, K.; Leine, D.; Blume, A. Colloids Surf. B: Biointerfaces 2006, 49, 31. [30] Johnson, I.; Olofsson, G.; Jönsson, B. J. Chem. Soc., Faraday Trans. 1 1987, 83, 3331. [31] Király, Z.; Dekány, I. J. Colloid Interface Sci. 2001, 242, 214. [32] Bhattacharya, S.; Haldar, J. Langmuir 2005, 21, 5747. [33] Mosquera, V.; del Río, J. M.; Attwood, D.; García, M.; Jones, M. N.; Prieto, G.; Suarez, M. J.; Sarmiento, F. J. Colloid Interface Sci. 1998, 206, 66. [34] Ford, D. M. J. Am. Chem. Soc. 2005, 127, 16167. [35] Starikov, E. B.; Nordén, B. J. Phys. Chem. B 2007, 111, 14431. [36] Rodríguez, J. R.; González-Pérez, A.; Del Castillo, J. L.; Czapkiewicz, J. J. Colloid Interface Sci. 2002, 250, 438. [37] Muller, N. Langmuir 1993, 9, 96. [38] Jolicoeur, C.; Philip, P. R. Can. J. Chem. 1974, 52, 1834. [39] Bai, G.; Wang, J.; Yan, H.; Li, Z.; Thomas, R. K. J. Phys. Chem. B 2001, 105, 9576. [40] Loladze, V. V.; Ermolenko, D. N.; Makhatadze, G. I. Protein Sci. 2001, 10, 1343. [41] Bergqvist, S.; Williams, M. A.; O'Brien, R.; Ladbury, J. E. J. Mol. Biol. 2004, 336, 829. [42] Spolar, R. S.; Livingstone, J. R.; Thomas Record, M. Biochemistry 1992, 31, 3947. [43] Lee, B.; Richards, F. M. J. Mol. Biol. 1971, 55, 379. [44] Richards, F. M. J. Mol. Biol. 1974, 82, 1. [45] Garidel, P.; Hildebrand, A.; Neubert, R.; Blume, A. Langmuir 2000, 16, 5267. [46] Majhi, P. R.; Blume, A. J. Phys. Chem. B 2002, 106, 10753. [47] Majhi, P. R.; Blume, A. Langmuir 2001, 17, 3844. [48] Mathias, J. H.; Rosen, M. J.; Davenport, L. Langmuir 2001, 17, 6148. |