化学学报 ›› 2022, Vol. 80 ›› Issue (8): 1057-1060.DOI: 10.6023/A22030130 上一篇 下一篇
研究通讯
投稿日期:2022-03-24
发布日期:2022-09-01
通讯作者:
薛敏
基金资助:
Minggang Wua, Yong Yangb, Min Xuea( )
)
Received:2022-03-24
Published:2022-09-01
Contact:
Min Xue
Supported by:文章分享
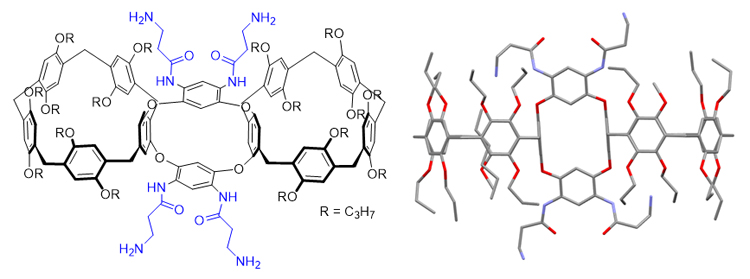
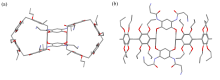
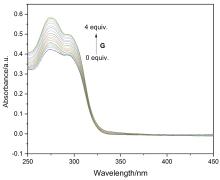
构象固定的刚性多环主体分子为构筑高级复杂的机械互锁结构提供了重要平台. 为挑战合成刚性多环主体并进一步构筑多层次机械互锁结构, 氧杂杯[4]芳烃桥连的柱[5]芳烃二聚体经过Raney Ni催化氢化还原硝基、与叔丁氧羰基(Boc)-β-丙氨酸缩合和脱去N-Boc保护基三步反应, 生成了四氨基柱[5]芳烃二聚体. X射线单晶衍射实验表明三环目标主体分子具有双桶望远镜形状, 并且构象刚性, 随取代基不同仅有微小变化. 此外, 该四氨基二聚体可作为主体与己二腈形成高稳定性的1∶2络合物. 该研究为制备复杂超分子体系提供了新的机会.
吴明港, 杨勇, 薛敏. 四氨基柱[5]芳烃二聚体的合成、结构及性质研究[J]. 化学学报, 2022, 80(8): 1057-1060.
Minggang Wu, Yong Yang, Min Xue. Tetraaminopillar[5]arene Dimers: Synthesis, Structure and Properties[J]. Acta Chimica Sinica, 2022, 80(8): 1057-1060.




| [1] |
(a) Diederich, F.; Stang, P. J.; Tykwinski, R. R. Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis, Wiley-VCH, Berlin, 2008, p. 1.
|
|
(b) Steed, J. W.; Atwood, J. L. Supramolecular Chemistry, John Wiley & Sons, Ltd., New York, 2009, p. 1.
|
|
|
(c) Li, J.; Han, Y.; Chen, C. Chin. J. Org. Chem. 2020, 40, 3714. (in Chinese)
doi: 10.6023/cjoc202005007 |
|
|
(李晶, 韩莹, 陈传峰, 有机化学, 2020, 40, 3714.)
doi: 10.6023/cjoc202005007 |
|
| [2] |
(a) Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Chem. Rev. 2015, 115, 7398.
doi: 10.1021/cr5005869 |
|
(b) Xia, D.; Wang, P.; Ji, X.; Khashab, N. M.; Sessler, J. L.; Huang, F. Chem. Rev. 2020, 120, 6070.
doi: 10.1021/acs.chemrev.9b00839 |
|
|
(c) Han, X. N.; Han, Y.; Chen, C. F. J. Am. Chem. Soc. 2020, 142, 8262.
doi: 10.1021/jacs.0c00624 |
|
| [3] |
(a) Jiang, Y.; Guo, J. B.; Chen, C. F. Chem. Commun. 2010, 46, 5536.
doi: 10.1039/c0cc00999g |
|
(b) Evans, N. H.; Serpell, C. J.; Beer, P. D. Angew. Chem., Int. Ed. 2011, 50, 2507.
doi: 10.1002/anie.201007741 |
|
|
(c) Li, C.; Han, K.; Li, J.; Zhang, H.; Ma, J.; Shu, X.; Chen, Z.; Weng, L.; Jia, X. Org. Lett. 2012, 14, 42.
doi: 10.1021/ol2027834 |
|
|
(d) Hu, W. B.; Xie, C. D.; Hu, W. J.; Zhao, X. L.; Liu, Y. A.; Huo, J. C.; Li, J. S.; Jiang, B.; Wen, K. J. Org. Chem. 2015, 80, 7994.
doi: 10.1021/acs.joc.5b01038 |
|
|
(e) Zhou, Y.; Jie, K.; Shi, B.; Yao, Y. Chem. Commun. 2015, 51, 11112.
doi: 10.1039/C5CC02886H |
|
|
(f) Sun, Y.; Wang, J.; Yao, Y. Chem. Commun. 2017, 53, 165.
doi: 10.1039/C6CC08452D |
|
|
(g) Ding, Y.; Alimi, L. O.; Moosa, B.; Maaliki, C.; Jacquemin, J.; Huang, F.; Khashab, N. M. Chem. Sci. 2021, 12, 5315.
doi: 10.1039/D1SC00440A |
|
| [4] |
(a) Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1397.
doi: 10.1002/anie.201006693 |
|
(b) Song, N.; Chen, D. X.; Qiu, Y. C.; Yang, X. Y.; Xu, B.; Tian, W.; Yang, Y. W. Chem. Commun. 2014, 50, 8231.
doi: 10.1039/c4cc03105a |
|
|
(c) Meng, L. B.; Li, D.; Xiong, S.; Hu, X. Y.; Wang, L.; Li, G. Chem. Commun. 2015, 51, 4643.
doi: 10.1039/C5CC00398A |
|
|
(d) Huo, B.; Li, B.; Su, H.; Zeng, X.; Xu, K.; Cui, L. Chin. J. Org. Chem. 2019, 39, 1990. (in Chinese)
doi: 10.6023/cjoc201811003 |
|
|
(霍博超, 李斌, 苏杭, 曾宪强, 徐凯迪, 崔雷, 有机化学, 2019, 39, 1990.)
doi: 10.6023/cjoc201811003 |
|
|
(e) Xiao, T.; Zhou, L.; Sun, X.-Q.; Huang, F.; Lin, C.; Wang, L. Chin. Chem. Lett. 2020, 31, 1.
doi: 10.1016/j.cclet.2019.05.011 |
|
| [5] |
(a) Wu, J.; Sun, S.; Feng, X.; Shi, J.; Hu, X. Y.; Wang, L. Chem. Commun. 2014, 50, 9122.
doi: 10.1039/C4CC03127J |
|
(b) Yue, S. Y.; Zhou, Y. J.; Yao, Y.; Xue, M. Acta Chim. Sinica 2014, 72, 1053. (in Chinese)
doi: 10.6023/A14080579 |
|
|
(岳诗雨, 周玉娟, 姚勇, 薛敏, 化学学报, 2014, 72, 1053.)
doi: 10.6023/A14080579 |
|
|
(c) Wang, Q.; Cheng, M.; Zhao, Y.; Wu, L.; Jiang, J.; Wang, L.; Pan, Y. Chem. Commun. 2015, 51, 3623.
doi: 10.1039/C5CC00130G |
|
|
(d) Song, N.; Chen, D. X.; Xia, M. C.; Qiu, X. L.; Ma, K.; Xu, B.; Tian, W.; Yang, Y. W. Chem. Commun. 2015, 51, 5526.
doi: 10.1039/C4CC08205B |
|
|
(e) Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J. L.; Meng, Q.; Li, C. Chem. Sci. 2020, 11, 6275.
doi: 10.1039/D0SC01756F |
|
| [6] |
(a) Wu, X.; Duan, Q. P.; Ni, M. F.; Hu, X. Y.; Wang, L. Y. Chin. J. Org. Chem. 2014, 34, 437. (in Chinese)
|
|
(吴旋, 段群鹏, 倪梦飞, 胡晓玉, 王乐勇, 有机化学, 2014, 34, 437.)
doi: 10.6023/cjoc201312018 |
|
|
(b) Hu, W. B.; Hu, W. J.; Zhao, X. L.; Liu, Y. A.; Li, J. S.; Jiang, B.; Wen, K. Chem. Commun. 2015, 51, 13882.
doi: 10.1039/C5CC05623C |
|
|
(c) Li, S. H.; Zhang, H. Y.; Xu, X.; Liu, Y. Nat. Commun. 2015, 6, 7590.
doi: 10.1038/ncomms8590 |
|
|
(d) Zhang, R.; Wang, C.; Sun, J.; Yan, C.; Yao, Y. Chin. J. Org. Chem. 2019, 39, 3483. (in Chinese)
doi: 10.6023/cjoc201906006 |
|
|
(张润淼, 王陈威, 孙晶, 颜朝国, 姚勇, 有机化学, 2019, 39, 3483.)
doi: 10.6023/cjoc201906006 |
|
|
(e) Liang, H.; Hua, B.; Xu, F.; Gan, L. S.; Shao, L.; Huang, F. J. Am. Chem. Soc. 2020, 142, 19772.
doi: 10.1021/jacs.0c10570 |
|
|
(f) Dong, J. H.; Li, J. J.; Wang, H.; Liu, B. X.; Peng, B.; Chen, J. Z.; Lin, S. L. Acta Chim. Sinica 2021, 79, 803. (in Chinese)
doi: 10.6023/A21030105 |
|
|
(董锦辉, 李进杰, 王赫, 刘彬秀, 彭博, 陈健壮, 林绍梁, 化学学报, 2021, 79, 803.)
doi: 10.6023/A21030105 |
|
| [7] |
(a) Li, Z. Y.; Zhang, Y.; Zhang, C. W.; Chen, L. J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H. B. J. Am. Chem. Soc. 2014, 136, 8577.
doi: 10.1021/ja413047r |
|
(b) Chen, J.; Liu, X.; Han, B.; Ding, J.; Zhang, Y.; Lin, Q.; Yao, H.; Wei, T. Chin. J. Org. Chem. 2018, 38, 2741. (in Chinese)
doi: 10.6023/cjoc201805003 |
|
|
(陈进发, 刘茜, 韩冰冰, 丁金东, 张有明, 林奇, 姚虹, 魏太保, 有机化学, 2018, 38, 2741.)
doi: 10.6023/cjoc201805003 |
|
|
(c) Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Angew. Chem., Int. Ed. 2019, 58, 3885.
doi: 10.1002/anie.201813972 |
|
|
(d) Liu, J.; Sun, X. W.; Huang, T. T.; Zhang, Y. M.; Yao, H.; Wei, T. B.; Lin, Q. Chin. J. Chem. 2021, 39, 3421.
doi: 10.1002/cjoc.202100583 |
|
| [8] |
(a) Si, W.; Chen, L.; Hu, X. B.; Tang, G.; Chen, Z.; Hou, J. L.; Li, Z. T. Angew. Chem., Int. Ed. 2011, 50, 12564.
doi: 10.1002/anie.201106857 pmid: 23362942 |
|
(b) Chen, L.; Si, W.; Zhang, L.; Tang, G.; Li, Z. T.; Hou, J. L. J. Am. Chem. Soc. 2013, 135, 2152.
doi: 10.1021/ja312704e pmid: 23362942 |
|
|
(c) Yan, Z. J.; Wang, D.; Ye, Z.; Fan, T.; Wu, G.; Deng, L.; Yang, L.; Li, B.; Liu, J.; Ma, T.; Dong, C.; Li, Z. T.; Xiao, L.; Wang, Y.; Wang, W.; Hou, J. L. J. Am. Chem. Soc. 2020, 142, 15638.
doi: 10.1021/jacs.0c00601 pmid: 23362942 |
|
|
(d) Yan, Z. J.; Li, Y. W.; Yang, M.; Fu, Y. H.; Wen, R.; Wang, W.; Li, Z. T.; Zhang, Y.; Hou, J. L. J. Am. Chem. Soc. 2021, 143, 11332.
doi: 10.1021/jacs.1c06000 pmid: 23362942 |
|
|
(e) Li, Y. W.; Fu, Y. H.; Hou, J. L. Chin. J. Chem. 2022, 40, 1293.
doi: 10.1002/cjoc.202100836 pmid: 23362942 |
|
| [9] |
Wan, K.; Gao, S. C.; Fang, X.; Xu, M. Y.; Yang, Y.; Xue, M. Chem. Commun. 2020, 56, 10155.
doi: 10.1039/D0CC04375C |
| [10] |
Shu, X.; Chen, S.; Li, J.; Chen, Z.; Weng, L.; Jia, X.; Li, C. Chem. Commun. 2012, 48, 2967.
doi: 10.1039/c2cc00153e |
| [11] |
(a) Thordarson, P. Chem. Soc. Rev. 2010, 40, 1305.
doi: 10.1039/C0CS00062K |
|
(b) Hibbert, D. B.; Thordarson, P. Chem. Commun. 2016, 52, 12792.
doi: 10.1039/C6CC03888C |
|
| [12] |
Capici, C.; Gattuso, G.; Notti, A.; Parisi, M. F.; Pappalardo, S.; Brancatelli, G.; Geremia, S. J. Org. Chem. 2012, 77, 9668.
doi: 10.1021/jo301730m |
| [1] | 黄秀清, 张琦. 葫芦状有机金属配位笼的合成及其对两种药物分子的选择性结合[J]. 化学学报, 2023, 81(3): 217-221. |
| [2] | 魏颖, 周平, 陈鑫, 包秋景, 解令海. 有机纳米环/格的研究进展[J]. 化学学报, 2023, 81(3): 289-308. |
| [3] | 田小茂, 林悦群, 朱菡, 黄超, 朱必学. 手性单Schiff碱大环对青霉胺对映体识别研究[J]. 化学学报, 2023, 81(1): 20-28. |
| [4] | 刘传志, 李芬, 王静静, 赵晓璐, 张婷美, 黄鑫, 邬梦丽, 户志远, 刘新明, 黎占亭. 基于分子间卤键的超分子平面大环自组装[J]. 化学学报, 2022, 80(10): 1365-1368. |
| [5] | 闫腾飞, 刘盛达, 罗逸尘, 邹应萍, 刘俊秋. 大环分子衍生物在人工跨膜离子通道领域中的研究进展[J]. 化学学报, 2021, 79(8): 999-1007. |
| [6] | 周丽, 徐立进, 龚汉元. 自组装中的苯二甲酸阴离子形貌及质子化效应[J]. 化学学报, 2014, 72(4): 447-455. |
| [7] | 岳诗雨, 周玉娟, 姚勇, 薛敏. 柱芳烃化学:从合成、主客体性质到自组装性能的研究进展[J]. 化学学报, 2014, 72(10): 1053-1069. |
| [8] | 付云, 王海蛟, 张骥, 余孝其. 大环多胺-咪唑鎓盐阳离子脂质的设计合成及作为基因载体的性质研究[J]. 化学学报, 2013, 71(04): 585-592. |
| [9] | 韩成友, 张子彬, 池小东, 张明明, 喻国灿, 黄飞鹤. 1,4-双正丙氧基柱[7]芳烃的合成及主客体化学[J]. 化学学报, 2012, 70(17): 1775-1778. |
| [10] | 杨登科, 曾志坚, 陈木娟, 潘绍武, 杨宇, 李媚, 雷春燕, 蒋腊生. 二酰胺大环化合物与吡啶N-氧化物的络合作用研究[J]. 化学学报, 2012, 70(12): 1385-1393. |
| [11] | 宋玉民, 张玉梅, 马新贤, 朱早龙, 许军鹏, 刘景旺. 姜黄素稀土大环配合物的光致变色性能研究[J]. 化学学报, 2011, 69(11): 1347-1353. |
| [12] | 张颖, 王欣, 李来才. 一种非血红素四氮杂轮烯配合物[Fe(III)TMTAA]催化过氧化氢歧化反应机理的理论研究[J]. 化学学报, 2010, 68(07): 633-640. |
| [13] | 胡学雷,a,b 陈 中a 邱 立a 刘 波b 赵元弟b 潘志权a 罗勤慧c. 双酚大环配体硝酸铕三元配合物的合成、晶体结构与荧光性质[J]. 化学学报, 2008, 66(12): 1446-1450. |
| [14] | 段志勇, 张家仲, 许杏祥. Sarsolilide A的合成研究——十四元碳环骨架的构建[J]. 化学学报, 2004, 62(8): 811-817. |
| [15] | 郝爱友,王金山. 用环糊精合成楔筒状主体分子新模型[J]. 化学学报, 2002, 60(8): 1536-1538. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||