Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (9): 1238-1249.DOI: 10.6023/A22050227 Previous Articles Next Articles
Article
李小娟, 叶梓瑜, 谢书涵, 王永净, 王永好, 吕源财, 林春香*( )
)
投稿日期:2022-05-16
发布日期:2022-08-11
通讯作者:
林春香
基金资助:
Xiaojuan Li, Ziyu Ye, Shuhan Xie, Yongjing Wang, Yonghao Wang, Yuancai Lv, Chunxiang Lin( )
)
Received:2022-05-16
Published:2022-08-11
Contact:
Chunxiang Lin
Supported by:Share
Xiaojuan Li, Ziyu Ye, Shuhan Xie, Yongjing Wang, Yonghao Wang, Yuancai Lv, Chunxiang Lin. Study on Performance and Mechanism of Phenol Degradation through Peroxymonosulfate Activation by Nitrogen/Chlorine Co-doped Porous Carbon Materials[J]. Acta Chimica Sinica, 2022, 80(9): 1238-1249.
| Sample | SSAa/ (m2•g-1) | TPVb/ (cm3•g-1) | APS b/ nm | Vmicro c/ (cm3•g-1) | Vmeso c/ (cm3•g-1) |
|---|---|---|---|---|---|
| NC | 1060.82 | 0.23 | 3.76 | 0.06 | 0.17 |
| NC900 | 1418.02 | 0.40 | 3.27 | 0.12 | 0.28 |
| NClC700 | 1101.09 | 0.24 | 3.49 | 0.07 | 0.13 |
| NClC800 | 1530.69 | 0.42 | 2.86 | 0.14 | 0.28 |
| NClC900 | 2259.60 | 0.84 | 2.96 | 0.25 | 0.59 |
| Sample | SSAa/ (m2•g-1) | TPVb/ (cm3•g-1) | APS b/ nm | Vmicro c/ (cm3•g-1) | Vmeso c/ (cm3•g-1) |
|---|---|---|---|---|---|
| NC | 1060.82 | 0.23 | 3.76 | 0.06 | 0.17 |
| NC900 | 1418.02 | 0.40 | 3.27 | 0.12 | 0.28 |
| NClC700 | 1101.09 | 0.24 | 3.49 | 0.07 | 0.13 |
| NClC800 | 1530.69 | 0.42 | 2.86 | 0.14 | 0.28 |
| NClC900 | 2259.60 | 0.84 | 2.96 | 0.25 | 0.59 |
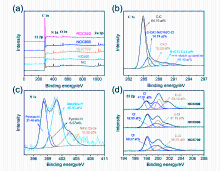

| Sample | C | N | O | Cl | Zn | C=O | 吡啶N | 吡咯N | 石墨N | 氧化N |
|---|---|---|---|---|---|---|---|---|---|---|
| NC | 64.00 | 15.05 | 6.87 | — | 14.08 | 12.43 | 32.89 | 26.32 | 27.63 | 13.16 |
| NC900 | 77.85 | 7.15 | 7.55 | — | 7.45 | 11.51 | 36.21 | 5.17 | 43.10 | 15.52 |
| NClC700 | 62.94 | 12.36 | 7.51 | 1.53 | 15.66 | 11.73 | 46.51 | 13.49 | 28.84 | 11.16 |
| NClC800 | 68.57 | 8.35 | 10.36 | 2.31 | 10.41 | 8.39 | 42.73 | 10.26 | 34.62 | 12.39 |
| NClC900 | 83.59 | 4.33 | 7.09 | 1.10 | 3.59 | 10.26 | 31.46 | 6.57 | 46.95 | 15.02 |
| Sample | C | N | O | Cl | Zn | C=O | 吡啶N | 吡咯N | 石墨N | 氧化N |
|---|---|---|---|---|---|---|---|---|---|---|
| NC | 64.00 | 15.05 | 6.87 | — | 14.08 | 12.43 | 32.89 | 26.32 | 27.63 | 13.16 |
| NC900 | 77.85 | 7.15 | 7.55 | — | 7.45 | 11.51 | 36.21 | 5.17 | 43.10 | 15.52 |
| NClC700 | 62.94 | 12.36 | 7.51 | 1.53 | 15.66 | 11.73 | 46.51 | 13.49 | 28.84 | 11.16 |
| NClC800 | 68.57 | 8.35 | 10.36 | 2.31 | 10.41 | 8.39 | 42.73 | 10.26 | 34.62 | 12.39 |
| NClC900 | 83.59 | 4.33 | 7.09 | 1.10 | 3.59 | 10.26 | 31.46 | 6.57 | 46.95 | 15.02 |

| 猝灭剂 | ROS | 抑制率/ % | ROS贡献值 (A) | ROS贡献率(A/T, %) |
|---|---|---|---|---|
| 50/2000 mmol/L MeOH | (SO4•-+•OH)S | 2.6/2.6 | SO4•-S=1.3 | SO4•-S=0.9 |
| 50/2000 mmol/L TBA | •OHS+C | 1.3/5.7 | •OHS=1.3 •OHC=4.4 | •OHS=0.9 •OHC=3.1 |
| 20 mmol/L KI | (SO4•-+•OH)C | 61.2 | SO4•-C=56.8 | SO4•-C=40.6 |
| 20 mmol/L L-histidine | 1O2 | 55.0 | 1O2=55.0 | 1O2=39.3 |
| 20 mmol/L Na2CO3 | O2•- | 21.3 | O2•-=21.3 | O2•-=15.2 |
| 猝灭剂 | ROS | 抑制率/ % | ROS贡献值 (A) | ROS贡献率(A/T, %) |
|---|---|---|---|---|
| 50/2000 mmol/L MeOH | (SO4•-+•OH)S | 2.6/2.6 | SO4•-S=1.3 | SO4•-S=0.9 |
| 50/2000 mmol/L TBA | •OHS+C | 1.3/5.7 | •OHS=1.3 •OHC=4.4 | •OHS=0.9 •OHC=3.1 |
| 20 mmol/L KI | (SO4•-+•OH)C | 61.2 | SO4•-C=56.8 | SO4•-C=40.6 |
| 20 mmol/L L-histidine | 1O2 | 55.0 | 1O2=55.0 | 1O2=39.3 |
| 20 mmol/L Na2CO3 | O2•- | 21.3 | O2•-=21.3 | O2•-=15.2 |
| [1] |
Ganiyu S. O.; Sable S.; Gamal El-Din M. Chem. Eng. J. 2022, 429, 132492.
|
| [2] |
Liu L.; Chen Z.; Zhang J.; Shan D.; Wu Y.; Bai L.; Wang B. J Water Process Eng. 2021, 42, 102122.
|
| [3] |
Luo H.; Fu H.; Yin H.; Lin Q. J. Hazard. Mater. 2022, 426, 128044.
doi: 10.1016/j.jhazmat.2021.128044 |
| [4] |
Chen X.; Oh W.; Lim T. Chem. Eng. J. 2018, 354, 941.
doi: 10.1016/j.cej.2018.08.049 |
| [5] |
Luo R.; Wu J.; Zhao J.; Fang D.; Liu Z.; Hu L. Environ. Res. 2022, 204, 112060.
doi: 10.1016/j.envres.2021.112060 |
| [6] |
Xie J.; Chen L.; Luo X.; Huang L.; Li S.; Gong X. Sep. Purif. Technol. 2022, 281, 119887.
doi: 10.1016/j.seppur.2021.119887 |
| [7] |
Humphrey N.; Rodriguez R.; Arias G.; Thai E.; Muro E.; Merinov B. V.; Goddard W. A.; Yu T. H. J. Catal. 2020, 381, 295.
doi: 10.1016/j.jcat.2019.10.022 |
| [8] |
Zhang C.; Bai J.; Ma L.; Lv Y.; Wang F.; Zhang X.; Yuan X.; Hu S. Diam. Relat. Mater. 2018, 87, 215.
doi: 10.1016/j.diamond.2018.06.013 |
| [9] |
Wu Q.; Liang J.; Yi J.; Shi P.; Huang Y.; Cao R. Science China Materials 2018, 62, 671.
doi: 10.1007/s40843-018-9364-5 |
| [10] |
Wang N.; Ma W.; Ren Z.; Zhang L.; Qiang R.; Lin K.-Y. A.; Xu P.; Du Y.; Han X. Inorg. Chem. Front. 2018, 5, 1849.
doi: 10.1039/C8QI00256H |
| [11] |
Ferrari A. C.; Robertson J. Phys. Rev. B 2000, 61, 14095.
doi: 10.1103/PhysRevB.61.14095 |
| [12] |
Dresselhaus M. S.; Dresselhaus G.; Saito R.; Jorio A. Phys. Rep. 2005, 409, 47.
doi: 10.1016/j.physrep.2004.10.006 |
| [13] |
Zhang M.; Luo R.; Wang C.; Zhang W.; Yan X.; Sun X.; Wang L.; Li J. J. Mater. Chem. A 2019, 7, 12547.
doi: 10.1039/c9ta02931a |
| [14] |
Yang Y.; Jin H.; Zhang C.; Gan H.; Yi F.; Wang H. J. Alloys Compd. 2020, 821, 153439.
doi: 10.1016/j.jallcom.2019.153439 |
| [15] |
Tang L.; Liu Y.; Wang J.; Zeng G.; Deng Y.; Dong H.; Feng H.; Wang J.; Peng B. Appl. Catal., B 2018, 231, 1.
|
| [16] |
Huang B.; Jiang J.; Huang G.; Yu H. J. Mater. Chem. A 2018, 6, 8978.
doi: 10.1039/C8TA02282H |
| [17] |
Zhang J.; Chen P.; Gao W.; Wang W.; Tan F.; Wang X.; Qiao X.; Wong P. K. Sep. Purif. Technol. 2021, 265, 118474.
doi: 10.1016/j.seppur.2021.118474 |
| [18] |
Chen F.; Cheng X.; Zhao Z.; Wang X. Acta Chim. Sinica 2021, 79, 941.(in Chinese)
doi: 10.6023/A21030117 |
|
(陈峰, 程晓琴, 赵振新, 王晓敏, 化学学报, 2021, 79, 941.)
|
|
| [19] |
Wang G.; Chen S.; Quan X.; Yu H.; Zhang Y. Carbon 2017, 115, 730.
doi: 10.1016/j.carbon.2017.01.060 |
| [20] |
Cheng Z.; Zheng K.; Lin G.; Fang S.; Li L.; Bi J.; Shen J.; Wu L. Nanoscale Adv. 2019, 1, 2674.
doi: 10.1039/C9NA00089E |
| [21] |
Bianco G. V.; Sacchetti A.; Milella A.; Grande M.; D’orazio A.; Capezzuto P.; Bruno G. Carbon 2020, 170, 75.
doi: 10.1016/j.carbon.2020.07.038 |
| [22] |
Ghanbari F.; Moradi M. Chem. Eng. J. 2017, 310, 41.
doi: 10.1016/j.cej.2016.10.064 |
| [23] |
Abdul Nasir Khan M.; Kwame Klu P.; Wang C.; Zhang W.; Luo R.; Zhang M.; Qi J.; Sun X.; Wang L.; Li J. Chem. Eng. J. 2019, 363, 234.
doi: 10.1016/j.cej.2019.01.129 |
| [24] |
Ma W.; Wang N.; Tong T.; Zhang L.; Lin K. A.; Han X.; Du Y. Carbon 2018, 137, 291.
doi: 10.1016/j.carbon.2018.05.039 |
| [25] |
Guan Y.; Ma J.; Ren Y.; Liu Y.; Xiao J.; Lin L.; Zhang C. Water Res. 2013, 47, 5431.
doi: 10.1016/j.watres.2013.06.023 |
| [26] |
Deng Z.; Yang X.; Xu W. Journal of Tongji University Natural Science, 2009, 37, 354.(in Chinese)
|
|
(邓子峰, 杨晓, 徐伟, 同济大学学报(自然科学版), 2009, 37, 354.)
|
|
| [27] |
Zhang L.; Kanki T.; Sano N.; Toyoda A. Environ. Monit. Assess. 2006, 115, 395.
doi: 10.1007/s10661-006-7236-y |
| [28] |
Liang J.; Xu X.; Qamar Zaman W.; Hu X.; Zhao L.; Qiu H.; Cao X. Chem. Eng. J. 2019, 375, 121908.
doi: 10.1016/j.cej.2019.121908 |
| [29] |
Wang Q.; Li L.; Luo L.; Yang Y.; Yang Z.; Li H.; Zhou Y. Chem. Eng. J. 2019, 376, 120891.
doi: 10.1016/j.cej.2019.01.170 |
| [30] |
Wu L.; Yu Y.; Zhang Q.; Hong J.; Wang J.; She Y. Appl. Surf. Sci. 2019, 480, 717.
doi: 10.1016/j.apsusc.2019.03.034 |
| [31] |
Xi T.; Li X.; Zhang Q.; Liu N.; Niu S.; Dong Z.; Lyu C. Front. Env. Sci. Eng. 2021, 15, 11.
doi: 10.1007/s11783-020-1303-4 |
| [32] |
Cheng X.; Guo H.; Zhang Y.; Wu X.; Liu Y. Water Res. 2017, 113, 80.
doi: S0043-1354(17)30091-X pmid: 28199865 |
| [33] |
Duan W.; He J.; Wei Z.; Dai Z.; Feng C. Environ. Sci.: Nano 2020, 7, 2982.
doi: 10.1039/D0EN00848F |
| [34] |
Pang K.; Sun W.; Ye F.; Yang L.; Pu M.; Yang C.; Zhang Q.; Niu J. J. Hazard. Mater. 2022, 424, 127270.
doi: 10.1016/j.jhazmat.2021.127270 |
| [35] |
Gul I.; Sayed M.; Shah N. S.; Rehman F.; Khan J. A.; Gul S.; Bibi N.; Iqbal J. Environ. Sci. Pollut. Res. 2021, 28, 23368.
doi: 10.1007/s11356-020-11497-2 |
| [36] |
Lyu L.; Yu G.; Zhang L.; Hu C.; Sun Y. Environ. Sci. Technol. 2018, 52, 747.
doi: 10.1021/acs.est.7b04865 |
| [37] |
Li J.; He L.; Jiang J.; Xu Z.; Liu M.; Liu X.; Tong H.; Liu Z.; Qian D. Electrochim. Acta 2020, 353, 136579.
doi: 10.1016/j.electacta.2020.136579 |
| [38] |
Ma Y.; Liu R.; Meng S.; Niu L.; Yang Z.; Lei Z. Acta Chim. Sinica 2019, 77, 153.(in Chinese)
doi: 10.6023/A18090372 |
|
(马亚丽, 刘茹雪, 孟双艳, 牛力同, 杨志旺, 雷自强, 化学学报, 2019, 77, 153.)
|
|
| [39] |
Liu Y.; Miao W.; Fang X.; Tang Y.; Wu D.; Mao S. Chem. Eng. J. 2020, 380, 122584.
doi: 10.1016/j.cej.2019.122584 |
| [40] |
Guo W.; Yu J.; Dai Z.; Hou W. Acta Chim. Sinica 2019, 77, 1203.(in Chinese)
doi: 10.6023/A19080316 |
|
(郭文娟, 于洁, 代昭, 侯伟钊, 化学学报, 2019, 77, 1203.)
doi: 10.6023/A19080316 |
| [1] | Yang Liu, Fengqin Gao, Zhanying Ma, Yinli Zhang, Wuwu Li, Lei Hou, Xiaojuan Zhang, Yaoyu Wang. Co-based Metal-organic Framework for High-efficiency Degradation of Methylene Blue in Water by Peroxymonosulfate Activation [J]. Acta Chimica Sinica, 2024, 82(2): 152-159. |
| [2] | Bo Sun, Wenwen Ju, Tao Wang, Xiaojun Sun, Ting Zhao, Xiaomei Lu, Feng Lu, Quli Fan. Preparation of Highly-dispersed Conjugated Polymer-Metal Organic Framework Nanocubes for Antitumor Application [J]. Acta Chimica Sinica, 2023, 81(7): 757-762. |
| [3] | Junchang Chen, Mingxing Zhang, Shuao Wang. Research Progress of Synthesis Methods for Crystalline Porous Materials [J]. Acta Chimica Sinica, 2023, 81(2): 146-157. |
| [4] | Xu Yan, Hemi Qu, Ye Chang, Xuexin Duan. Application of Metal-Organic Frameworks in Gas Pre-concentration, Pre-separation and Detection [J]. Acta Chimica Sinica, 2022, 80(8): 1183-1202. |
| [5] | Fang Liu, Tingting Pan, Xiurong Ren, Weiren Bao, Jiancheng Wang, Jiangliang Hu. Research on Preparation and Benzene Adsorption Performance of HCDs@MIL-100(Fe) Adsorbents [J]. Acta Chimica Sinica, 2022, 80(7): 879-887. |
| [6] | Linan Cao, Min Wei. Recent Progress of Electric Conductive Metal-Organic Frameworks Thin Film [J]. Acta Chimica Sinica, 2022, 80(7): 1042-1056. |
| [7] | Shihui Wang, Xiaoyu Xue, Min Cheng, Shaochen Chen, Chong Liu, Li Zhou, Kexin Bi, Xu Ji. High-Throughput Computational Screening of Metal-Organic Frameworks for CH4/H2 Separation by Synergizing Machine Learning and Molecular Simulation [J]. Acta Chimica Sinica, 2022, 80(5): 614-624. |
| [8] | Rong Zhang, Jiangping Liu, Ziyi Zhu, Shumei Chen, Fei Wang, Jian Zhang. Synthesis, Structure and Characterization of Two Ferrocene Functionalized Cadmium Metal Organic Frameworks※ [J]. Acta Chimica Sinica, 2022, 80(3): 249-254. |
| [9] | Xusheng Wang, Xu Yang, Chunhui Chen, Hongfang Li, Yuanbiao Huang, Rong Cao. Graphene Quantum Dots Supported on Fe-based Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction※ [J]. Acta Chimica Sinica, 2022, 80(1): 22-28. |
| [10] | Yan-Wu Zhao, Xing Li, Fu-Qiang Zhang, Xiang Zhang. Precise Control of the Dimension of Homochiral Metal-Organic Frameworks (MOFs) and Their Luminescence Properties [J]. Acta Chimica Sinica, 2021, 79(11): 1409-1414. |
| [11] | Huan Liu, Li Li, Ping Li, Guangzhi Zhang, Xun Xu, Hao Zhang, Lingfang Qiu, Hui Qi, Shuwang Duo. In-situ Construction of 2D/3D ZnIn2S4/TiO2 with Enhanced Photocatalytic Performance [J]. Acta Chimica Sinica, 2021, 79(10): 1293-1301. |
| [12] | Sun Lian, Wang Honglei, Yu Jinshan, Zhou Xingui. Recent Progress on Proton-Conductive Metal-Organic Frameworks and Their Proton Exchange Membranes [J]. Acta Chimica Sinica, 2020, 78(9): 888-900. |
| [13] | Wu Qianye, Zhang Chenxi, Sun Kang, Jiang Hai-Long. Microwave-Assisted Synthesis and Photocatalytic Performance of a Soluble Porphyrinic MOF [J]. Acta Chimica Sinica, 2020, 78(7): 688-694. |
| [14] | Zhang Jinwei, Li Ping, Zhang Xinning, Ma Xiaojie, Wang Bo. Water Adsorption Properties and Applications of Stable Metal-organic Frameworks [J]. Acta Chimica Sinica, 2020, 78(7): 597-612. |
| [15] | Chen Yang, Du Yadan, Wang Yong, Liu Puxu, Li Libo, Li Jinping. Ammonia Modification on UTSA-280 for C2H4/C2H6 Separation [J]. Acta Chimica Sinica, 2020, 78(6): 534-539. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||