Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (7): 879-887.DOI: 10.6023/A22020074 Previous Articles Next Articles
Article
刘芳a, 潘婷婷a, 任秀蓉a,b, 鲍卫仁a,b,c, 王建成a,b, 胡江亮a,b,c,*( )
)
投稿日期:2022-02-18
发布日期:2022-05-05
通讯作者:
胡江亮
基金资助:
Fang Liua, Tingting Pana, Xiurong Rena,b, Weiren Baoa,b,c, Jiancheng Wanga,b, Jiangliang Hua,b,c( )
)
Received:2022-02-18
Published:2022-05-05
Contact:
Jiangliang Hu
Supported by:Share
Fang Liu, Tingting Pan, Xiurong Ren, Weiren Bao, Jiancheng Wang, Jiangliang Hu. Research on Preparation and Benzene Adsorption Performance of HCDs@MIL-100(Fe) Adsorbents[J]. Acta Chimica Sinica, 2022, 80(7): 879-887.

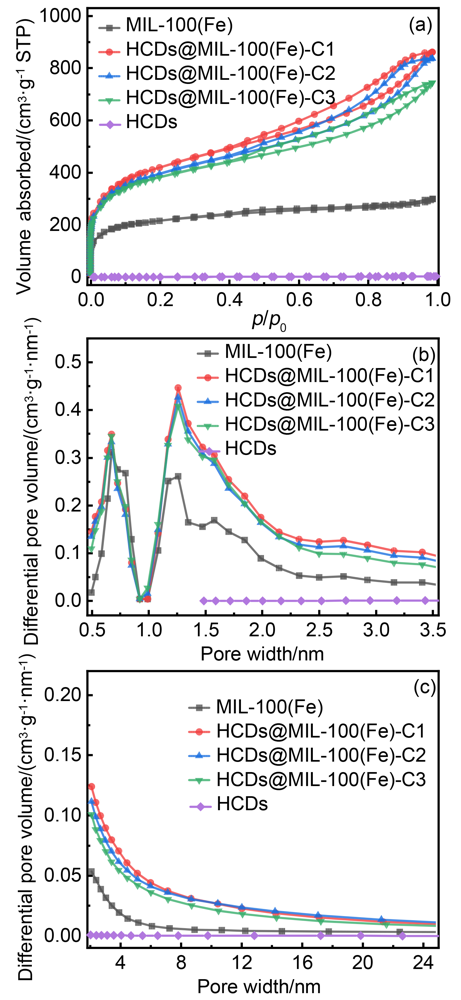
| Sample | ABET/(m2•g–1) | Pore volume/(cm3•g–1) | ||
|---|---|---|---|---|
| Vtotal | Vmeso | Vmicro | ||
| HCDs | 3.70 | 0.0053 | 0.0050 | 0.0003 |
| MIL-100(Fe) | 786.21 | 0.46 | 0.24 | 0.22 |
| HCDs@MIL-100(Fe)-C1 | 1546.97 | 1.33 | 0.98 | 0.35 |
| HCDs@MIL-100(Fe)-C2 | 1458.87 | 1.29 | 0.96 | 0.33 |
| HCDs@MIL-100(Fe)-C3 | 1406.97 | 1.15 | 0.83 | 0.32 |
| Sample | ABET/(m2•g–1) | Pore volume/(cm3•g–1) | ||
|---|---|---|---|---|
| Vtotal | Vmeso | Vmicro | ||
| HCDs | 3.70 | 0.0053 | 0.0050 | 0.0003 |
| MIL-100(Fe) | 786.21 | 0.46 | 0.24 | 0.22 |
| HCDs@MIL-100(Fe)-C1 | 1546.97 | 1.33 | 0.98 | 0.35 |
| HCDs@MIL-100(Fe)-C2 | 1458.87 | 1.29 | 0.96 | 0.33 |
| HCDs@MIL-100(Fe)-C3 | 1406.97 | 1.15 | 0.83 | 0.32 |

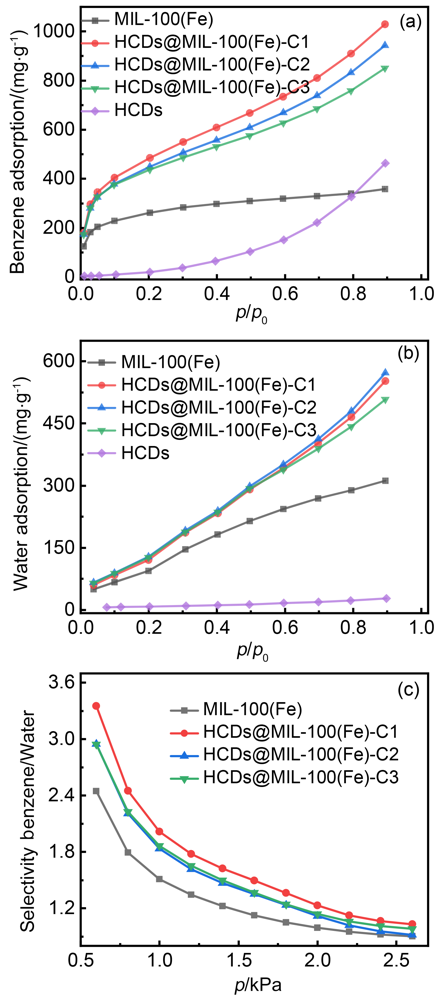
| 吸附剂 | 吸附量/(mg•g–1) | ||||
|---|---|---|---|---|---|
| p/p0=0.1 | p/p0=0.9 | ||||
| 苯 | 水 | 苯 | 水 | ||
| HCDs | 0.31 | 2.47 | 461.57 | 22.97 | |
| MIL-100(Fe) | 226.06 | 62.38 | 356.10 | 307.79 | |
| HCDs@MIL-100(Fe)-C1 | 401.71 | 79.25 | 1029.23 | 549.57 | |
| HCDs@MIL-100(Fe)-C2 | 375.81 | 84.78 | 942.77 | 569.52 | |
| HCDs@MIL-100(Fe)-C3 | 372.54 | 83.62 | 850.58 | 505.13 | |
| 吸附剂 | 吸附量/(mg•g–1) | ||||
|---|---|---|---|---|---|
| p/p0=0.1 | p/p0=0.9 | ||||
| 苯 | 水 | 苯 | 水 | ||
| HCDs | 0.31 | 2.47 | 461.57 | 22.97 | |
| MIL-100(Fe) | 226.06 | 62.38 | 356.10 | 307.79 | |
| HCDs@MIL-100(Fe)-C1 | 401.71 | 79.25 | 1029.23 | 549.57 | |
| HCDs@MIL-100(Fe)-C2 | 375.81 | 84.78 | 942.77 | 569.52 | |
| HCDs@MIL-100(Fe)-C3 | 372.54 | 83.62 | 850.58 | 505.13 | |

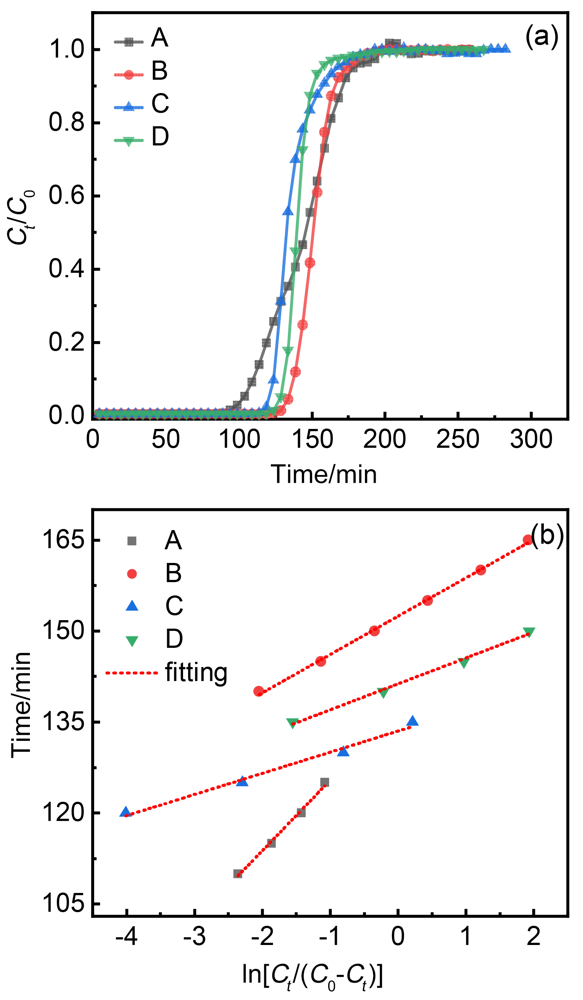
| 吸附剂 | k/min–1 |
|---|---|
| MIL-100(Fe) | 0.0861 |
| HCDs@MIL-100(Fe)-C1 | 0.1583 |
| HCDs@MIL-100(Fe)-C2 | 0.2869 |
| HCDs@MIL-100(Fe)-C3 | 0.2342 |
| 吸附剂 | k/min–1 |
|---|---|
| MIL-100(Fe) | 0.0861 |
| HCDs@MIL-100(Fe)-C1 | 0.1583 |
| HCDs@MIL-100(Fe)-C2 | 0.2869 |
| HCDs@MIL-100(Fe)-C3 | 0.2342 |
| [1] |
Wang, X.; Wu, Y.-S.; Yang, X.; Chen, H.-Y.; Zhang, J.-B.; Ma, X.-X. Chem. Ind. Eng. Prog. 2021, 40, 2813. (in Chinese)
|
|
(王旭, 吴玉帅, 杨欣, 陈汇勇, 张建波, 马晓迅, 化工进展, 2021, 40, 2813.)
|
|
| [2] |
Zhang, Z.; Jiang, Z.; Shangguan, W. Catal. Today 2016, 264, 270.
doi: 10.1016/j.cattod.2015.10.040 |
| [3] |
Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Chem. Eng. J. 2019, 370, 1128.
doi: 10.1016/j.cej.2019.03.232 |
| [4] |
Wang, X.-C.; Wang, S.; Liu, D.-X. Environ. Pollut. Control 2020, 42, 1387. (in Chinese)
|
|
(王学臣, 王帅, 刘大喜, 环境污染与防治, 2020, 42, 1387.)
|
|
| [5] |
Zhu, M.; Tong, Z.; Zhao, Z.; Jiang, Y.; Zhao, Z. Ind. Eng. Chem. Res. 2016, 55, 3765.
doi: 10.1021/acs.iecr.6b00056 |
| [6] |
Li, L.; Liu, S.; Liu, J. J. Hazard. Mater. 2011, 192, 683.
doi: 10.1016/j.jhazmat.2011.05.069 |
| [7] |
Dai, J.; Zhao, C.; Hu, X.; Chen, D.; Yun, J.; Liu, C.; Zhong, M.; Huang, D.; Cen, C.; Chen, Z. J. Chem. Technol. Biotechnol. 2021, 96, 78.
|
| [8] |
Zhou, L.; Zhang, X.; Chen, Y. Mater. Lett. 2017, 197, 167.
doi: 10.1016/j.matlet.2017.03.162 |
| [9] |
Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Sep. Purif. Technol. 2020, 235, 116213.
doi: 10.1016/j.seppur.2019.116213 |
| [10] |
Zhang, H.; Li, G.-L.; Zhang, K.-G.; Liao, C.-Y. Acta Chim. Sinica 2017, 75, 9. (in Chinese)
|
|
(张贺, 李国良, 张可刚, 廖春阳, 化学学报, 2017, 75, 9.)
|
|
| [11] |
Yang, K.; Xue, F.; Sun, Q.; Yue, R.; Lin, D. J. Environ. Chem. Eng. 2013, 1, 713.
doi: 10.1016/j.jece.2013.07.005 |
| [12] |
Jayaramulu, K.; Geyer, F.; Schneemann, A.; Kment, S.; Otyepka, M.; Zboril, R.; Vollmer, D.; Fischer, R. A. Adv. Mater. 2019, 31, 1900820.
|
| [13] |
Xian, S.; Yu, Y.; Xiao, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. RSC Adv. 2014, 5, 18274.
|
| [14] |
Zhang, J.-W.; Li, P.; Zhang, X.-N.; Ma, X.-J.; Wang, B. Acta Chim. Sinica 2020, 78, 597. (in Chinese)
doi: 10.6023/A20050153 |
|
(张晋维, 李平, 张馨凝, 马小杰, 王博, 化学学报, 2020, 78, 597.)
doi: 10.6023/A20050153 |
|
| [15] |
Szczesniak, B.; Choma, J.; Jaroniec, M. Microporous Mesoporous Mater. 2019, 279, 387.
doi: 10.1016/j.micromeso.2019.01.022 |
| [16] |
Ahsan, M. A.; Jabbari, V.; Islam, M. T.; Turley, R. S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J. A.; Curry, M. L.; Lopez, J. Sci. Total Environ. 2019, 673, 306.
doi: 10.1016/j.scitotenv.2019.03.219 |
| [17] |
Li, M.; Huang, W.; Tang, B.; Song, F.; Ling, X. J. Nanomater. 2019, 2019, 1.
|
| [18] |
Xia, Z.; Shi, B.; Zhu, W.; Lü, C. Chem. Eng. J. 2021, 426, 131794.
doi: 10.1016/j.cej.2021.131794 |
| [19] |
Sun, X.; Lv, D.; Chen, Y.; Wu, Y.; Wu, Q.; Xia, Q.; Li, Z. Energy Fuels 2017, 31, 13985.
doi: 10.1021/acs.energyfuels.7b02665 |
| [20] |
Han, T.; Xiao, Y.; Tong, M.; Huang, H.; Liu, D.; Wang, L.; Zhong, C. Chem. Eng. J. 2015, 275, 134.
doi: 10.1016/j.cej.2015.04.005 |
| [21] |
Horcajada, P.; Surble, S.; Serre, C.; Hong, D. Y.; Seo, Y. K.; Chang, J. S.; Greneche, J. M.; Margiolaki, I.; Ferey, G. Chem. Commun. 2007, 27, 2820.
|
| [22] |
Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Adv. Sci. 2019, 6, 1901316.
doi: 10.1002/advs.201901316 |
| [23] |
Mei, L.; Jiang, T.; Zhou, X.; Li, Y.; Wang, H.; Li, Z. Chem. Eng. J. 2017, 321, 600.
doi: 10.1016/j.cej.2017.03.131 |
| [24] |
Zhou, X.; Huang, W.; Shi, J.; Zhao, Z.; Xia, Q.; Li, Y.; Wang, H.; Li, Z. J. Mater. Chem. A 2014, 2, 4722.
doi: 10.1039/C3TA15086K |
| [25] |
Ma, X.; Wang, W.; Sun, C.; Li, H.; Sun, J.; Liu, X. Sci. Total Environ. 2021, 793, 148622.
doi: 10.1016/j.scitotenv.2021.148622 |
| [26] |
Chen, H.; Yuan, X.; Jiang, L.; Wang, H.; Zeng, G. Sep. Purif. Technol. 2022, 286, 120409.
doi: 10.1016/j.seppur.2021.120409 |
| [27] |
Alivand, M. S.; Tehrani, N. H. M. H.; Askarieh, M.; Ghasemy, E.; Esrafili, M. D.; Ahmadi, R.; Anisi, H.; Tavakoli, O.; Rashidi, A. J. Hazard. Mater. 2021, 416, 125973.
doi: 10.1016/j.jhazmat.2021.125973 |
| [28] |
Liu, Y.; Xia, X.-X.; Tan, Y.-Y.; Li, S. Acta Chim. Sinica 2020, 78, 250. (in Chinese)
doi: 10.6023/A19120449 |
|
(刘洋, 夏潇潇, 谭媛元, 李松, 化学学报, 2020, 78, 250.)
doi: 10.6023/A19120449 |
|
| [29] |
Sun, X.; Gu, X.; Xu, W.; Chen, W. J.; Xia, Q.; Pan, X.; Zhao, X.; Li, Y.; Wu, Q. H. Front. Chem. 2019, 7, 652.
doi: 10.3389/fchem.2019.00652 |
| [30] |
Chen, F.-L. M.S. Thesis, East China University of Science and Technology, Shanghai, 2016. (in Chinese)
|
|
(程方亮, 硕士论文,华东理工大学, 上海, 2016.)
|
|
| [31] |
Cheng, F.; An, X.; Zheng, C.; Cao, S. RSC Adv. 2015, 5, 93360.
doi: 10.1039/C5RA19029K |
| [32] |
Fan, X.; Hu, H.; Qin, J.; Cao, X.; Zhao, R.; Wang, D. Environ. Technol. Innovation 2021, 24, 101809.
doi: 10.1016/j.eti.2021.101809 |
| [33] |
Zhang, Y.; Liu, X.-Y.; Mao, H.-L.; Wang, C.; Du, H.; Cheng, H.; Zhuang, J.-L. J. Mater. Eng. 2019, 47, 71. (in Chinese)
|
|
(张宇, 刘湘粤, 毛会玲, 王晨, 杜嬛, 程琥, 庄金亮, 材料工程, 2019, 47, 71.)
|
|
| [34] |
Li, X.-M. M.S. Thesis, South China University of Technology, Guangzhou, 2013. (in Chinese)
|
|
(李雪梅, 硕士论文,华南理工大学, 广州, 2013.)
|
|
| [35] |
Zhao, Z.; Wang, S.; Yang, Y.; Li, X.; Li, J.; Li, Z. Chem. Eng. J. 2015, 259, 79.
doi: 10.1016/j.cej.2014.08.012 |
| [36] |
Li, J.; Kan, L.; Li, J.; Liu, Y. Angew. Chem., Int. Ed. 2020, 59, 19659.
doi: 10.1002/anie.202006978 |
| [37] |
Wang, G.; Li, N.; Xing, X.; Sun, Y.; Hao, Z. Chemosphere 2020, 247, 125862.
doi: 10.1016/j.chemosphere.2020.125862 |
| [38] |
Zhu, M.; Hu, P.; Tong, Z.; Zhao, Z.; Zhao, Z. Chem. Eng. J. 2017, 313, 1122.
doi: 10.1016/j.cej.2016.11.008 |
| [1] | Hangqing Lin, Ruoru Ma, Yilan Jiang, Murong Xu, Yangpeng Lin, Kezhao Du. Research Progress of Materials Used for Elemental Halogen Capture [J]. Acta Chimica Sinica, 2024, 82(1): 62-74. |
| [2] | Yuchun Han, Yilin Wang. Retrospect and Prospect of Long-lasting Antibacterial Materials★ [J]. Acta Chimica Sinica, 2023, 81(9): 1196-1201. |
| [3] | Ziqi Li, Liwei Liu, Chenghui Mao, Changkai Zhou, Minqi Xia, Zhen Shen, Yue Guo, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Cobalt-Substituted Polyoxometalates as Soluble Mediators to Boost the Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2023, 81(6): 620-626. |
| [4] | Kaiqing Wang, Shuo Yuan, Wangdong Xu, Dan Huo, Qiulin Yang, Qingxi Hou, Dehai Yu. Preparation and Adsorption Properties of ZIF-8@B-CNF Composite Aerogel [J]. Acta Chimica Sinica, 2023, 81(6): 604-612. |
| [5] | Shaojuan Zeng, Xueqi Sun, Yinge Bai, Lu Bai, Shuang Zheng, Xiangping Zhang, Suojiang Zhang. Research Progress of CO2 Capture and Separation by Functionalized Ionic Liquids and Materials★ [J]. Acta Chimica Sinica, 2023, 81(6): 627-645. |
| [6] | Zhao Zhenxin, Yao Yikun, Chen Jiajun, Niu Rong, Wang Xiaomin. A High-entropy Phosphate Cathode Host towards High-stability Lithium-sulfur Batteries [J]. Acta Chimica Sinica, 2023, 81(5): 496-501. |
| [7] | Jiangmin Jiang, Xinran Zheng, Yating Meng, Wenjie He, Yaxin Chen, Quanchao Zhuang, Jiaren Yuan, Zhicheng Ju, Xiaogang Zhang. Research on the Preparation and Potassium Storage Performance of F, N Co-doped Porous Carbon Nanosheets [J]. Acta Chimica Sinica, 2023, 81(4): 319-327. |
| [8] | Wentao Wang, Xinting Lai, Shiquan Yan, Lei Zhu, Yuyuan Yao, Liming Ding. Synergistic Treatment of Dye Wastewater by the Adsorption-Degradation of a Bifunctional Aerogel [J]. Acta Chimica Sinica, 2023, 81(3): 222-230. |
| [9] | Bing Zheng, Zhe Wang, Jing He, Jiao Zhang, Wenbo Qi, Mengyuan Zhang, Haitao Yu. Structure and Work Function of Alkaline (Earth) Metal-Bilayer α-Borophene Nanocomposite: A Theoretical Study [J]. Acta Chimica Sinica, 2023, 81(10): 1357-1370. |
| [10] | Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※ [J]. Acta Chimica Sinica, 2022, 80(5): 640-646. |
| [11] | Junrui Liu, Jinglin Chen, Jie Yang, Xiaofeng Xu, Ruonan Li, You-Gui Huang, Shaohua Chen, Xin Ye, Wei Wang. K+-Site Ce-Doped Jarosite for Phosphate Adsorption: a Mechanism Study※ [J]. Acta Chimica Sinica, 2022, 80(4): 476-484. |
| [12] | Yaru Wei, Jing Ma, Tingting Yuan, Jiawei Jiang, Yinli Duan, Juanqin Xue. Preparation and Adsorption Properties of Lithium Chloride Intercalation Carbon Nitride [J]. Acta Chimica Sinica, 2022, 80(4): 494-502. |
| [13] | Rong Zhang, Jiangping Liu, Ziyi Zhu, Shumei Chen, Fei Wang, Jian Zhang. Synthesis, Structure and Characterization of Two Ferrocene Functionalized Cadmium Metal Organic Frameworks※ [J]. Acta Chimica Sinica, 2022, 80(3): 249-254. |
| [14] | Chaofeng Wang, Guodong Zheng, Yue Wang, Huijia Song, Xiaoyi Chen, Ruixia Gao. Preparation of Controllable Non-covalent Functionalized Carbon Nanotubes with Metalloporphyrin-Sn Network and Application to Protein Adsorption [J]. Acta Chimica Sinica, 2022, 80(2): 126-132. |
| [15] | Yarui Song, Kaisheng Wang, Guangyu An, Fajun Zhao, Bin Men, Zhaoxi Du, Dongsheng Wang. Preparation of Powdered Activated Carbon Matrix Composites and Their Decontamination Performance and Mechanisms for Oily Sewage [J]. Acta Chimica Sinica, 2022, 80(12): 1592-1599. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||