Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (10): 999-1007.DOI: 10.6023/A19060233 Previous Articles Next Articles
Article
投稿日期:2019-06-25
发布日期:2019-07-17
通讯作者:
曹鑫,俞飚
E-mail:caox@fudan.edu.cn;byu@sioc.ac.cn
基金资助:
Shao, Wenboa, An, Quanlinb, Cao, Xinb*( ), Yu, Biaoa*(
), Yu, Biaoa*( )
)
Received:2019-06-25
Published:2019-07-17
Contact:
Cao, Xin,Yu, Biao
E-mail:caox@fudan.edu.cn;byu@sioc.ac.cn
Supported by:Share
Shao, Wenbo, An, Quanlin, Cao, Xin, Yu, Biao. Efficient Synthesis of Representative Flavone-7-O-Glycosides[J]. Acta Chimica Sinica, 2019, 77(10): 999-1007.
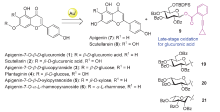
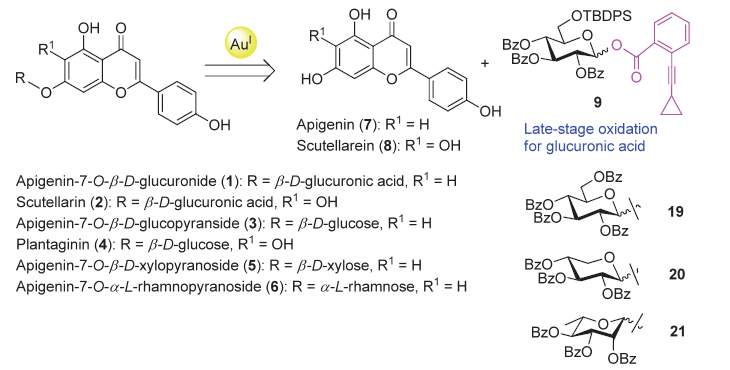
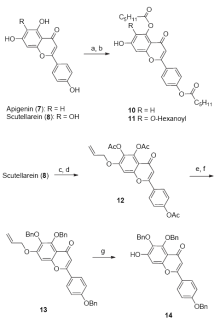


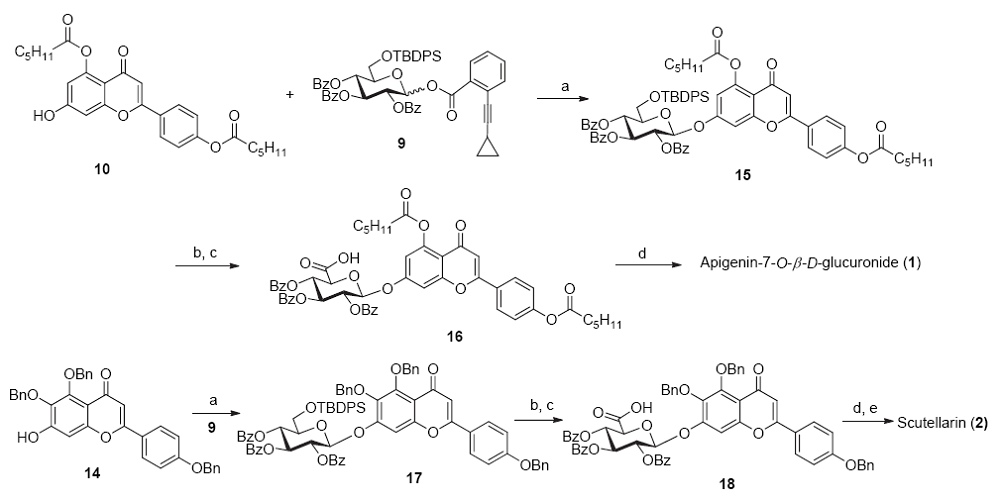
| [1] |
(a) Cui, J. M.; Wu, S . Nat. Prod. Res. Dev. 2003, 15, 255.
doi: 10.1016/j.jep.2018.05.022 |
|
(b) Ma, Y. H.; Luo, G. A.; Wang, Y. M . Chin. Tradit. Pat. Med. 2004, 1, 63.
doi: 10.1016/j.jep.2018.05.022 |
|
|
(c) Wang, J.; Zhang, L.; Liu, B.; Wang, Q.; Chen, Y.; Wang, Z.; Zhou, J.; Xiao, W.; Zheng, C.; Wang, Y . J. Ethnopharmacol. 2018, 224, 429.
doi: 10.1016/j.jep.2018.05.022 |
|
| [2] |
(a) Yue, J. M.; Lin, Z. W.; Wang, D. Z . Phytochemistry 1994, 36, 717.
doi: 10.1016/S0031-9422(00)89803-9 |
|
(b) Xia, H. J.; Qiu, F.; Zhu, S.; Zhang, T. Y.; Qu, G. X.; Yao, X. S . Biol. Pharm. Bull. 2007, 30, 1308.
doi: 10.1016/S0031-9422(00)89803-9 |
|
| [3] |
(a) Liu, Q.; Li, X.; Ouyang, X.; Chen, D . Molecules 2018, 23, 3225.
doi: 10.3390/molecules23123225 |
|
(b) Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y . Bioorg. Med. Chem. 2015, 23, 668.
doi: 10.3390/molecules23123225 |
|
| [4] |
(a) Wu, W. H.; Chen, T. Y.; Lu, R. W.; Chen, S. T.; Chang, C. C . Phytochemistry 2012, 83, 110.
doi: 10.1016/j.phytochem.2012.07.022 |
|
(b) Chen, V.; Staub, R. E.; Baggett, S.; Chimmani, R.; Tagliaferri, M.; Cohen, I.; Shtivelman, E . PLoS One 2012, 7, e30107.
doi: 10.1016/j.phytochem.2012.07.022 |
|
| [5] |
(a) Huang, X. W.; Xu, Y.; Sui, X.; Lin, H.; Xu, J. M.; Han, D.; Ye, D. D.; Lv, G. F.; Liu, Y. X.; Qu, X. B.; Duan, M. H . Oncol. Lett. 2019, 17, 5581.
doi: 10.1021/jm00283a001 |
|
(b) Li, H. M.; Gu, T.; Wu, W. Y.; Yu, S. P.; Fan, T. Y.; Zhong, Y.; Li, N. G . Med. Chem. 2018, 14, 1.
doi: 10.1021/jm00283a001 |
|
| [6] |
(a) Sherbeiny, E.; Ansari, E . Planta Med. 1976, 29, 129.
doi: 10.1055/s-0028-1097641 |
|
(b) Homberg, H.; Geiger, H . Phytochemistry 1980, 19, 2443.
doi: 10.1055/s-0028-1097641 |
|
|
(c) Smirnova, L. P.; GlyzinA, V. I.; Patudin, A. V.; Bankovskii, A. I . Chem. Nat. Compd. 1974, 10, 687.
doi: 10.1055/s-0028-1097641 |
|
|
(d) Shabrawy, M. O. A.; Hosni, H. A.; Garf, I. A.; Marzouk, M. M.; Kawashty, S. A.; Saleh, N. A. M . Biochem. Syst. Ecol. 2014, 56, 125.
doi: 10.1055/s-0028-1097641 |
|
| [7] |
(a) Jacobsson, M.; Malmberg, J.; Ellervik, U . Carbohydr. Res. 2006, 341, 1266.
doi: 10.1016/j.carres.2006.04.004 |
|
(b) Sun, J. S.; Laval, S.; Yu, B . Synthesis 2014, 46, 1030.
doi: 10.1016/j.carres.2006.04.004 |
|
|
(c) Li, Y.; Yang, W. Z.; Ma, Y.; Sun, J. S.; Shan, L.; Zhang, W. D.; Yu, B . Synlett 2011, 915.
doi: 10.1016/j.carres.2006.04.004 |
|
|
(d) Yang, W. Z.; Sun, J. S.; Yang, Z.; Han, W.; Zhang, W. D.; Yu, B . Tetrahedron Lett. 2012, 53, 2773.
doi: 10.1016/j.carres.2006.04.004 |
|
|
(e) Hu, Y.; Tu, Y. H.; Liu, D. Y.; Liao, J. X.; Sun, J. S . Org. Biomol. Chem. 2016, 14, 4842.
doi: 10.1016/j.carres.2006.04.004 |
|
|
(f) Liao, J. X.; Fan, N. L.; Liu, H.; Tu, Y. H.; Sun, J. S . Org. Biomol. Chem. 2016, 14, 1221.
doi: 10.1016/j.carres.2006.04.004 |
|
|
(g) Wang, Y.; Liu, M.; Liu, L.; Xia, J. H.; Du, Y. G.; Sun, J. S . J. Org. Chem. 2018, 83, 4111.
doi: 10.1016/j.carres.2006.04.004 |
|
| [8] |
(a) Farkas, L.; Mezey-Vandor, G.; Nogradi, M . Chem. Ber. 1971, 104, 2681.
doi: 10.1002/(ISSN)1099-0682 |
|
(b) Farkas, L.; Mezey-Vandor, G.; Nogradi, M . Chem. Ber. 1974, 107, 3874.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(c) Synthesis for 1: Li, P. H.; Zhang, Z. P.; Zhang, W.; Yang, Z. X . CN 104761599, 2015.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(d) Synthesis of 2: (i) Nagashima, S.; Hirotani, M.; Yoshikawa, T . Phytochemistry 2000, 53, 533.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(ii) Li, P. H.; Zhang, W.; Yang, Z. X.; Zhang, X. B.; Wang, J.; Zhu, H. B.; Chen, J. X.; Bai, Y. Y . EP 2840088, 2015.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(e) Synthesis of 3: (i) Nakaoki, N . Yakugaku Zasshi 1940, 60, 502.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(ii) Oyama, K. I.; Kondo, T . Tetrahedron 2004, 60, 2025.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(iii) Liu, J. D.; Chen, L.; Cai, S. L.; Wang, Q . Carbohydr. Res. 2012, 357, 41.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(iv) Zheng, Z. W.; Han, Z. Y.; Cai, L.; Zhou, D. D.; Chavis, B. R.; Li, C. S.; Sui, Q.; Jiang, K. Y.; Gao, Q . Tetrahedron Lett. 2018, 59, 4442.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(f) Synthesis of 4: Li, N. G.; Shen, M. Z.; Wang, Z. J.; Tang, Y. P.; Shi, Z. H.; Fu, Y. F.; Shi, Q. P.; Tang, H.; Duan, J. A . Bioorg. Med. Chem. Lett. 2013, 23, 102.
doi: 10.1002/(ISSN)1099-0682 |
|
| [9] |
(a) Li, Y.; Yang, Y.; Yu, B . Tetrahedron Lett. 2008, 49, 3604.
doi: 10.1016/j.tetlet.2008.04.017 |
|
(b) Li, Y.; Yang, X.; Liu, Y.; Zhu, C.; Yang, Y.; Yu, B . Chem. -Eur. J. 2010, 16, 1871.
doi: 10.1016/j.tetlet.2008.04.017 |
|
|
(c) Zhu, Y.; Yu, B . Angew. Chem., Int. Ed. 2011, 50, 8329.
doi: 10.1016/j.tetlet.2008.04.017 |
|
|
(d) Tang, Y.; Li, J.; Zhu, Y.; Li, Y.; Yu, B . J. Am. Chem. Soc. 2013, 135, 18396.
doi: 10.1016/j.tetlet.2008.04.017 |
|
|
(e) Li, W.; Yu, B . Chem. Soc. Rev. 2018, 47, 7954.
doi: 10.1016/j.tetlet.2008.04.017 |
|
|
(f) Yu, B . Acc. Chem. Res. 2018, 51, 507.
doi: 10.1016/j.tetlet.2008.04.017 |
|
| [10] |
(a) Zhu, D.; Yu, B . Chin. J. Chem. 2018, 36, 681.
doi: 10.1002/cjoc.v36.8 |
|
(b) Li, J.; Yu, B . Angew. Chem., Int. Ed. 2015, 54, 6618.
doi: 10.1002/cjoc.v36.8 |
|
|
(c) Bai, Y.; Shen, X.; Li, Y.; Dai, M . J. Am. Chem. Soc. 2016, 138, 10838.
doi: 10.1002/cjoc.v36.8 |
|
|
(d) Wang, B.; Liu, Y.; Jiao, R.; Feng, Y.; Li, Q.; Chen, C.; Liu, L.; He, G.; Chen, G . J. Am. Chem. Soc. 2016, 138, 3926.
doi: 10.1002/cjoc.v36.8 |
|
|
(e) Nicolaou, K. C.; Cai, Q.; Sun, H.; Qin, B.; Zhu, S . J. Am. Chem. Soc. 2016, 138, 3118.
doi: 10.1002/cjoc.v36.8 |
|
|
(f) Nie, S. Y.; Li, W.; Yu, B . J. Am. Chem. Soc. 2014, 136, 4157.
doi: 10.1002/cjoc.v36.8 |
|
|
(g) Zhang, X.; Zhou, Y.; Zuo, J.; Yu, B . Nat. Commun. 2015, 6, 5879.
doi: 10.1002/cjoc.v36.8 |
|
|
(h) Shen, R. Z.; Cao, X.; Yu, B . Acta Chim. Sinica 2018, 76, 278.
doi: 10.1002/cjoc.v36.8 |
|
|
( 沈仁增, 曹鑫, 俞飚, 化学学报, 2018, 76, 278.)
doi: 10.1002/cjoc.v36.8 |
|
| [11] |
(a) Yang, W. Z.; Sun, J. S.; Lu, W. X.; Li, Y.; Shan, L.; Han, W.; Zhang, W. D.; Yu, B . J. Org. Chem. 2010, 75, 6879.
doi: 10.1021/jo1014189 |
|
(b) Yang, W. Z.; Li R. Y.; Han, W.; Zhang, W. D.; Sun, J. S . Chin. J. Org. Chem. 2012, 32, 1067.
doi: 10.1021/jo1014189 |
|
|
( 杨为准, 李荣耀, 韩伟, 张卫东, 孙建松, 有机化学, 2012, 32, 1067.)
doi: 10.1021/jo1014189 |
|
| [12] |
Liu, X.; Wen, G. E.; Liu, J. C.; Liao, J. X.; Sun, J. S . Carbohydr. Res. 2019, 475, 69.
doi: 10.1016/j.carres.2019.02.005 |
| [13] | Karst, N.; Jean-Claude, J . J. Chem. Soc., Perkin Trans. 1 2000, 16, 2709. |
| [14] | Yu, J.; Sun, J. S.; Niu, Y. M.; Li, R. Y.; Liao, J. X.; Zhang, F. Y.; Yu, B. Chem. Sci . 2013, 4, 3899. |
| [15] |
(a) Zulueta, M. M. L.; Lin, S. Y.; Lin, Y. T.; Huang, C. J.; Wang, C. C.; Ku, C. C.; Shi, Z.; Chyan, C. L.; Irene, D.; Lim, L. H.; Tsai, T. I.; Hu, Y. P.; Arco, S. D.; Wong, C. H.; Hung, S. C . J. Am. Chem. Soc. 2012, 134, 8988.
doi: 10.1021/ja302640p |
|
(b) Chang, C. H.; Lico, L. S.; Huang, T. Y.; Lin, S. Y.; Chang, C. L.; Arco, S. D.; Hung, S. C . Angew. Chem., Int. Ed. 2014, 53, 9876.
doi: 10.1021/ja302640p |
|
| [16] |
Li, M.; Han, X. W.; Yu, B . J. Org. Chem. 2003, 68, 6842.
doi: 10.1021/jo034553e |
| [17] |
Gao, Q.; Lian, G. Y.; Lin, F. Carbohydr. Res. 2008, 344, 511.
doi: 10.1016/j.carres.2008.12.016 |
| [1] | Lingyue Yang, Yunting Li, Chao Shu. Research Progress on Sultine [J]. Acta Chimica Sinica, 2024, 82(2): 171-189. |
| [2] | Yi Wan, Jianghua He, Yuetao Zhang. Research Progress in Precision Polymerization of Polar Olefin Monomers by Lewis Pairs★ [J]. Acta Chimica Sinica, 2023, 81(9): 1215-1230. |
| [3] | Mei Hong, Jinqiang Gao, Tong Li, Shihe Yang. In-situ Etching Strategy for Manipulation of Hierarchical Zeolite and Its Application★ [J]. Acta Chimica Sinica, 2023, 81(8): 937-948. |
| [4] | Shaoyan Gan, Shengyu Zhong, Liting Wang, Lei Shi. Synthesis and Application of Organic Hypervalent Bromine Reagents [J]. Acta Chimica Sinica, 2023, 81(8): 1030-1042. |
| [5] | Wenshan Zheng, Guanbin Gao, Hao Deng, Taolei Sun. Room Temperature Synthesis and Near-infrared Fluorescence Performance Optimization of Ag2Se@Ag2S Core-shell Quantum Dots [J]. Acta Chimica Sinica, 2023, 81(7): 763-770. |
| [6] | Yandong Zhang, Shoufei Zhu. Perspective for Phosphine Ligands with Cyclopropane Backbone★ [J]. Acta Chimica Sinica, 2023, 81(7): 777-783. |
| [7] | Kanbinuer Nuermaimaiti, Chao Wang, Shiwei Luo, Abudu Rexit Abulikemu. Research on Selective Dehalogenation of α,α,α-Trihalogen (Chloro, Bromo) methyl Ketones Under Electrochemical Conditions [J]. Acta Chimica Sinica, 2023, 81(6): 582-587. |
| [8] | Li Liu, Gang Zheng, Guoqiang Fan, Hongguang Du, Jiajing Tan. Research Progress in Organic Reactions Involving 4-Acyl/Carbamoyl/Alkoxycarbonyl Substituted Hantzsch Esters [J]. Acta Chimica Sinica, 2023, 81(6): 657-668. |
| [9] | Wang Jun, Xu Xiaomei, Zhou Jiaolong, Zhao Yanan, Sun Xiuli, Tang Yong, He Sufang, Yang Hongmei. Synthesis of New Sulfur-free and Phosphorus-free Ether-ester and Study on Its Properties As Ashless Friction Modifier [J]. Acta Chimica Sinica, 2023, 81(5): 461-468. |
| [10] | Junchang Chen, Mingxing Zhang, Shuao Wang. Research Progress of Synthesis Methods for Crystalline Porous Materials [J]. Acta Chimica Sinica, 2023, 81(2): 146-157. |
| [11] | Yang Gao, Xuexin Zhang, Jinsheng Yu, Jian Zhou. Recent Advances in Catalytic Enantioselective Synthesis of α-Chiral Tertiary Azides★ [J]. Acta Chimica Sinica, 2023, 81(11): 1590-1608. |
| [12] | Xiaochen Wang, Zeyao Ji, Jian Liu, Bingfu Wang, Hui Jin, Lixin Zhang. Advances in Organocatalytic Asymmetric Reactions Involving Thioesters [J]. Acta Chimica Sinica, 2023, 81(1): 64-83. |
| [13] | Zhiping Chen, Yongle Meng, Jing Lu, Wenwu Zhou, Zhiyuan Yang, Anning Zhou. Preparation of Fe@Si/S-34 Catalysts and Its Catalytic Performance for Syngas to Olefins [J]. Acta Chimica Sinica, 2023, 81(1): 14-19. |
| [14] | Hongdan Zhang, Xinyu Lan, Peng Cheng. Advances in Hydroxyl Free Radical Assisted Synthesis of Zeolite [J]. Acta Chimica Sinica, 2023, 81(1): 100-110. |
| [15] | Zhenhua Wang, Cong Ma, Ping Fang, Haichao Xu, Tiansheng Mei. Advances in Organic Electrochemical Synthesis [J]. Acta Chimica Sinica, 2022, 80(8): 1115-1134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||