Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (7): 920-924.DOI: 10.6023/A21040130 Previous Articles Next Articles
Article
王赫男, 张安歌, 张仲, 田洪瑞, 岳倩, 赵雪, 鹿颖, 刘术侠*( )
)
投稿日期:2021-04-02
发布日期:2021-04-25
通讯作者:
刘术侠
基金资助:
He-Nan Wang, An-Ge Zhang, Zhong Zhang, Hong-Rui Tian, Qian Yue, Xue Zhao, Ying Lu, Shu-Xia Liu( )
)
Received:2021-04-02
Published:2021-04-25
Contact:
Shu-Xia Liu
Supported by:Share
He-Nan Wang, An-Ge Zhang, Zhong Zhang, Hong-Rui Tian, Qian Yue, Xue Zhao, Ying Lu, Shu-Xia Liu. Synthesis and Properties of a Series of Pure Inorganic Ionic Liquids Based on Rare Earth Cations and Polyoxometalates[J]. Acta Chimica Sinica, 2021, 79(7): 920-924.
| Ionic liquid | H2O (w) | x | σ/(mS•cm-1) | ECW/V |
|---|---|---|---|---|
| 1 | 20.69 | 40.38 | 10.87 | 1.6 |
| 2 | 18.66 | 35.52 | 13.30 | 1.9 |
| 3 | 19.02 | 36.38 | 12.65 | 1.7 |
| 4 | 15.93 | 29.39 | 13.96 | 1.7 |
| 5 | 14.76 | 26.91 | 14.24 | 1.8 |
| 6 | 17.49 | 32.96 | 13.25 | 1.6 |
| 7 | 17.98 | 34.28 | 12.04 | 1.8 |
| 8 | 20.1 | 39.42 | 10.63 | 1.8 |
| Ionic liquid | H2O (w) | x | σ/(mS•cm-1) | ECW/V |
|---|---|---|---|---|
| 1 | 20.69 | 40.38 | 10.87 | 1.6 |
| 2 | 18.66 | 35.52 | 13.30 | 1.9 |
| 3 | 19.02 | 36.38 | 12.65 | 1.7 |
| 4 | 15.93 | 29.39 | 13.96 | 1.7 |
| 5 | 14.76 | 26.91 | 14.24 | 1.8 |
| 6 | 17.49 | 32.96 | 13.25 | 1.6 |
| 7 | 17.98 | 34.28 | 12.04 | 1.8 |
| 8 | 20.1 | 39.42 | 10.63 | 1.8 |


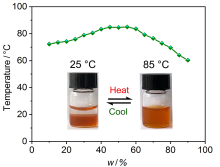
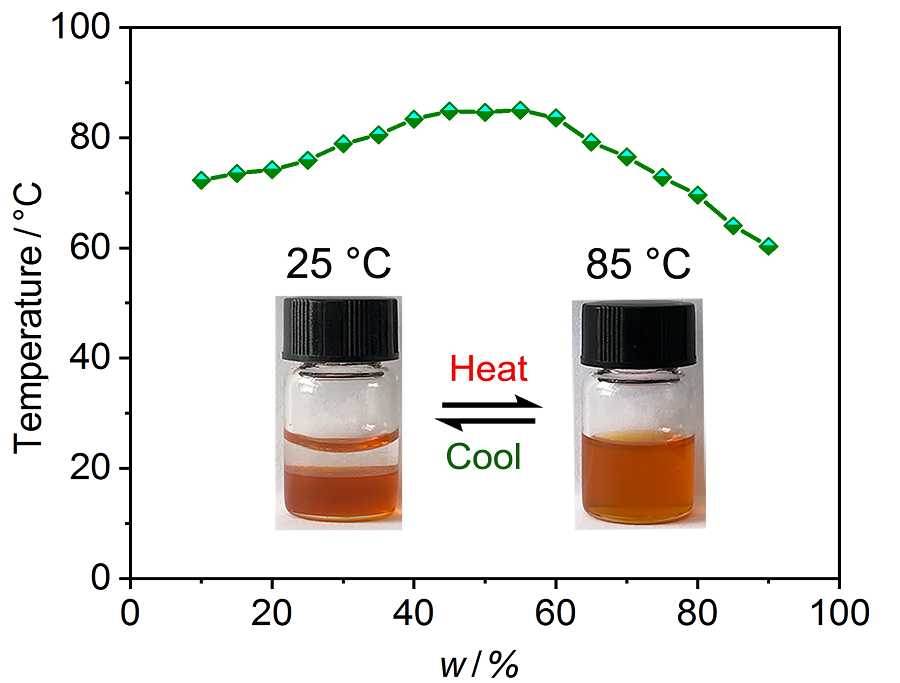
| [1] |
Hallett, J. P.; Welton, T. Chem. Rev. 2011, 111, 3508.
doi: 10.1021/cr1003248 pmid: 21469639 |
| [2] |
Lei, Z. G.; Chen, B. H.; Koo, Y. M.; MacFarlane, D. R. Chem. Rev. 2017, 117, 6633.
doi: 10.1021/acs.chemrev.7b00246 |
| [3] |
Rogers, R. D.; Seddon., K. R. Science 2003, 302, 792.
pmid: 14593156 |
| [4] |
Kang, X. C.; Sun, X. F.; Han, B. X. Adv. Mater. 2016, 28, 1011.
doi: 10.1002/adma.201502924 |
| [5] |
Li, S. N.; Zhao, W. X.; Liu, Y. J.; Liu, Z. Q.; Ying, A. G. Chin. J. Org. Chem. 2020, 40, 1835. (in Chinese)
doi: 10.6023/cjoc202003010 |
|
(李胜男, 赵雯辛, 刘玉静, 刘中秋, 应安国, 有机化学, 2020, 40, 1835.)
doi: 10.6023/cjoc202003010 |
|
| [6] |
Kore, R.; Berton, P.; Kelley, S. P.; Aduri, P.; Katti, S. S.; Rogers, R. D. ACS Catal. 2017, 7, 7014.
doi: 10.1021/acscatal.7b01793 |
| [7] |
Zhou, Z. H.; Chen, K. H.; He, L. N. Chin. J. Chem. 2019, 37, 1223.
doi: 10.1002/cjoc.v37.12 |
| [8] |
Yao, W. H.; Wang, H. Y.; Pei, Y. C.; Chen, Y. H.; Li, Z. Y.; Wang, J. Y. RSC Adv. 2017, 7, 11297.
doi: 10.1039/C6RA28483C |
| [9] |
Sun, T. X.; Shen, X. H.; Chen, Q. D. Acta Phys.-Chim. Sin. 2015, 31, 32. (in Chinese)
doi: 10.3866/PKU.WHXB201411051 |
|
(孙涛祥, 沈兴海, 陈庆德, 物理化学学报, 2015, 31, 32).
|
|
| [10] |
Bara, J. E.; Camper, D. E.; Gin, D. L.; Noble, R. D. Acc. Chem. Res. 2010, 43, 152.
doi: 10.1021/ar9001747 |
| [11] |
Xing, H. B.; Liao, C.; Yang, Q. W.; Veith, G. M.; Kun, G. B.; Sun, X. G.; Ren, Q. L.; Hu, Y. S.; Dai, S. Angew. Chem. Int. Ed. 2014, 53, 2099.
doi: 10.1002/anie.201309539 |
| [12] |
Kang, S. S.; Fan, S. C.; Liu, Y.; Wei, Y. C.; Li, Y.; Fang, J. G.; Meng, C. Z. Acta Chim. Sinica 2019, 77, 647. (in Chinese)
doi: 10.6023/A19040119 |
|
(康树森, 范少聪, 刘岩, 魏彦存, 李营, 房金刚, 孟垂舟, 化学学报, 2019, 77, 647).
doi: 10.6023/A19040119 |
|
| [13] |
Miras, H. N.; Yan, J.; Long, D. L.; Cronin, L. Chem. Soc. Rev. 2012, 41, 7403.
doi: 10.1039/c2cs35190k |
| [14] |
Wang, S. S.; Yang, G. Y. Chem. Rev. 2015, 115, 4893.
doi: 10.1021/cr500390v |
| [15] |
Wei, Z. Y.; Chang, Y. L.; Yu, H.; Han, S.; Wei, Y. G. Acta Chim. Sinica 2020, 78, 725. (in Chinese)
doi: 10.6023/A20050187 |
|
(魏哲宇, 常亚林, 余焓, 韩生, 魏永革, 化学学报, 2020, 78, 725).
|
|
| [16] |
Leng, Y.; Wang, J.; Zhu, D. R.; Ren, X. Q.; Ge, H. Q.; Shen, L. Angew. Chem. Int. Ed. 2009, 48, 168.
|
| [17] |
Herrmann, S.; Kostrzewa, M.; Wierschem, A.; Streb, C. Angew. Chem. Int. Ed. 2014, 53, 13596.
doi: 10.1002/anie.201408171 |
| [18] |
Qiao, Y. X.; Hou, Z. S.; Li, H.; Hu, Y.; Feng, B.; Wang, X. R.; Hua, L.; Huang, Q. F. Green Chem. 2009, 11, 1955.
doi: 10.1039/b916766h |
| [19] |
Dai, L. Y.; Yu, S. Y.; Shan, Y. K.; He, M. Y. Eur. J. Inorg. Chem. 2004,237.
|
| [20] |
Domaille, P. J.; Knoth, W. H. Inorg. Chem. 1983, 22, 818.
doi: 10.1021/ic00147a023 |
| [21] |
Bi, L. H.; Hussain, F.; Kortz, U.; Sadakane, M.; Dickman, M. H. Chem. Commun. 2004,1420.
|
| [22] |
Guan, W.; Yan, L. K.; Su, Z. M.; Liu, S. X.; Zhang, M.; Wang, X. H. Inorg. Chem. 2005, 44, 100.
pmid: 15627365 |
| [23] |
Wang, X. N.; Liu, S. X.; Li, S. J.; Xie, R. H.; Zhang, X.; Liu, Y. W. Chem. J. Chinese Universities. 2013, 34, 1047. (in Chinese)
|
|
(王雪娜, 刘术侠, 李书军, 谢瑞红, 张鑫, 刘艺伟, 高等学校化学学报, 2013, 34, 1047).
|
|
| [24] |
Wang, C. L.; Liu, S. X.; Xie, L. H.; Ren, Y. H.; Liang, D. D.; Sun, C. Y.; Cheng, H. Y. Polyhedron 2007, 26, 3017.
doi: 10.1016/j.poly.2007.02.005 |
| [25] |
Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Taguchi, S.; Liu, F.; Zeng, X.-B.; Ungar, G.; Ohno, H.; Kato, T. J. Am. Chem. Soc. 2012, 134, 2634.
doi: 10.1021/ja209010m |
| [26] |
Wu, X. F.; Zhou, X. H.; Wu, Q. Y.; Yan, W. F. New J. Chem. 2016, 40, 7923.
doi: 10.1039/C6NJ01205A |
| [27] |
Zheng, Q. G.; Liu, H.; Xia, Q.; Liu, Q. S.; Mou, L. Acta Phys.-Chim. Sin. 2017, 33, 736. (in Chinese)
doi: 10.3866/PKU.WHXB201612293 |
|
(郑其格, 刘惠, 夏泉, 刘青山, 牟林, 物理化学学报, 2017, 33, 736.)
|
|
| [28] |
Wang, C.; Ying, J.; Mou, H. C.; Tian, A. X.; Wang, X. L. Inorg. Chem. Front. 2020, 7, 3882.
doi: 10.1039/D0QI00505C |
| [29] |
Qiao, Y. X.; Ma, W. B.; Theyssen, N.; Chen, C.; Hou, Z. S. Chem. Rev. 2017, 117, 6881.
doi: 10.1021/acs.chemrev.6b00652 |
| [30] |
Depuydt, D.; Van den Bossche, A.; Dehaen, W.; Binnemans, K. Chem. Commun. 2017, 53, 5271.
doi: 10.1039/C7CC01685A |
| [31] |
Blesic, M.; Gunaratne, H. Q. N.; Jacquemin, J.; Nockemann, P.; Olejarz, S.; Seddon, K. R.; Strauss, C. R. Green Chem. 2014, 16, 4115.
doi: 10.1039/C4GC01159G |
| [32] |
Fukaya, Y.; Ohno, H. Phys. Chem. Chem. Phys. 2013, 15, 4066.
doi: 10.1039/c3cp44214d |
| [1] | Huimin Chen, Long Wang, Pan Zhang, Xilin Bai, Guojun Zhou. Investigation on Photoluminescence and Mechanoluminescence of Single Tb3+-doped Intense Green Phosphor [J]. Acta Chimica Sinica, 2023, 81(7): 771-776. |
| [2] | Yuewen Zhong, Xining Qian, Chao Ma, Kai Liu, Hongjie Zhang. Rare Earth Biological Manufacturing and High Value-added Material Application★ [J]. Acta Chimica Sinica, 2023, 81(11): 1624-1632. |
| [3] | Guanhua Zhang, Zihan Yang, Yue Ma. Effect of Mixing Strategy on Electrochemical Performance of Oxide/Sulfide Solid Electrolyte [J]. Acta Chimica Sinica, 2023, 81(10): 1387-1393. |
| [4] | Ling Qiu, Jiayi Liang, Zhuxia Zhang, Taishan Wang. Synthesis and Characterizations of 15N Isotope Labeling Metal Nitride Clusterfullerene [J]. Acta Chimica Sinica, 2022, 80(7): 874-878. |
| [5] | Xiaoke Hu, Xiaoying Shang, Ping Huang, Wei Zheng, Xueyuan Chen. Polarized Upconversion Luminescence from a Single NaYF4:Yb3+/Er3+ Microrod for Orientation Tracking※ [J]. Acta Chimica Sinica, 2022, 80(3): 244-248. |
| [6] | Xiangyu Zuo, Yifei Xu, Shikao Shi. Rare Earth Ions-Activated Hybrid Assemblies Fluorescent Systems Based on the Layered Lanthanum Hydroxides [J]. Acta Chimica Sinica, 2022, 80(2): 133-140. |
| [7] | Chenxiang Gong, Shuping Cheng, Xiangchuan Meng, Xiaotian Hu, Yiwang Chen. Recent Advances of PEDOT in Flexible Energy Conversion and Storage Devices [J]. Acta Chimica Sinica, 2021, 79(7): 853-868. |
| [8] | Yingzhe Du, Heng Zhang, Shiling Yuan. Molecular Dynamics Simulation of Thermal Conductivity of Al2O3/PDMS Composites [J]. Acta Chimica Sinica, 2021, 79(6): 787-793. |
| [9] | Huang Qingming. Study on the Upconversion Luminescence Mechanism of Tegtragonal LiYF4: RE with Sublattice Energy Cluster Construction and Crystal Field Manipulation [J]. Acta Chimica Sinica, 2020, 78(9): 968-979. |
| [10] | Guo Jinqiu, Du Yaping, Zhang Hongbo. A Brief Summary of Research Progress on the Application of Rare Earth Materials in Heterogeneous Catalysis [J]. Acta Chimica Sinica, 2020, 78(7): 625-633. |
| [11] | Jia Yiyi, Wang Wenjie, Liang Ling, Yuan Quan. Bioassay Applications of Aptamer-Functionalized Rare Earth Nanomaterials [J]. Acta Chimica Sinica, 2020, 78(11): 1177-1184. |
| [12] | Zhao Weichen, Xu Xin, Bai Huijuan, Zhang Jin, Lu Shanfu, Xiang Yan. Self-crosslinked Polyethyleneimine-polysulfone Membrane for High Temperature Proton Exchange Membrane [J]. Acta Chimica Sinica, 2020, 78(1): 69-75. |
| [13] | Wang Haixu, Yang Guang, Cheng Tianshu, Wang Ning, Sun Rong, Wong Ching-Ping. Recent Advances in Hydrothermal Synthesis of Low Dimensional Boron Nitride Nanostructures [J]. Acta Chim. Sinica, 2019, 77(4): 316-322. |
| [14] | Xiong Lin, Fan Yong, Zhang Fan. Research Progress on Rare Earth Nanocrystals for In Vivo Imaging and Sensing in Near Infrared Region [J]. Acta Chimica Sinica, 2019, 77(12): 1239-1249. |
| [15] | Nie Caina, Ma Xucun. Surface/Interface Issues of Superconducting Materials [J]. Acta Chim. Sinica, 2015, 73(7): 669-678. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||