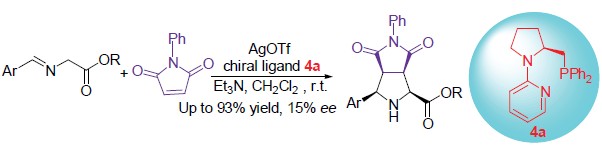
| [1] Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484. [2] Reviews for 1, 3-dipolar cycloaddition reaction: (a) Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765. (b) Najera, C.; Sansano, J. M. Angew. Chem., Int. Ed. 2005, 44, 6272. (c) 羖varez-Corral, M.; Muñoz-Dorado, M.; Rodr韌uez-Garc韆, I. Chem. Rev. 2008, 108, 3174. (d) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887; (e) Engels, B.; Christl, M. Angew. Chem., Int. Ed. 2009, 48, 7968. (f) Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784. [3] (a) Longmire, J. M.; Wang, B.; Zhang, X. J. Am. Chem. Soc. 2002, 124, 13400. (b) Gao, W.; Zhang, X.; Raghunath, M. Org. Lett. 2005, 7, 4241. [4] (a) Gothelf, A. S.; Gothelf, K. V.; Hazell, R. G.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2002, 41, 4236. (b) Alemparte, C.; Blay, G.; Jørgensen, K. A. Org. Lett. 2005, 7, 4569. [5] Chen, C.; Li, X.; Schreiber, S. L. J. Am. Chem. Soc. 2003, 125, 10174. [6] (a) Cabrera, S.; Array醩, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394. (b) Cabrera, S.; Array醩, R. G.; Martin-Matute, B.; Cossio, F. P.; Carretero, J. C. Tetrahedron 2007, 63, 6587. (c) Llamas, T.; Array醩, R. G.; Carretero, J. C. Org. Lett. 2006, 8, 1795. (d) Llamas, T.; Array醩, R. G.; Carretero, J. C. Synthesis 2007, 950. [7] (a) Zeng, W.; Chen, G.-Y.; Zhou, Y.-G.; Li, Y.-X. J. Am. Chem. Soc. 2007, 129, 750. (b) Zeng, W.; Zhou, Y.-G. Org. Lett. 2005, 7, 5055. (c) Zeng, W.; Zhou, Y.-G. Tetrahedron Lett. 2007, 48, 4619 [8] Yan, X.-X.; Peng, Q.; Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. Angew. Chem., Int. Ed. 2006, 45, 1979. [9] (a) N醞era, C.; De Gracia Retamosa, M.; Sansano, J. M. Org. Lett. 2007, 9, 4025. (b) N醞era, C.; De Gracia Retamosa, M.; Sansano, J. M. Angew. Chem., Int. Ed. 2008, 47, 6055 [10] Wang, C.-J.; Liang, G.; Xue, Z.-Y.; Gao, F. J. Am. Chem. Soc. 2008, 130, 17250. [11] Yamashita, Y.; Guo, X.-X.; Takashita, R.; Kobayashi, S. J. Am. Chem. Soc. 2010, 132, 3262. [12] Shi, M.; Shi, J.-W. Tetrahedron: Asymmetry 2007, 18, 645. [13] Arai, T., Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338. [14] (a) Nakagawa, Y.; Kanai, M.; Nagaoka, Y.; Tomioka, K. Tetrahedron 1998, 54, 10295. (b) Kanai, M.; Nakagawa, Y.; Tomioka, K. Tetrahedron 1999, 55, 3843. (c) Nakagawa, Y.; Matsumoto, K.; Tomioka, K. Tetrahedron 2000, 56, 2857.Gawley, R. E.; Hart, G. C.; Bartolotti, L. J. J. Org. Chem. 1989, 54, 175. |