有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2726-2734.DOI: 10.6023/cjoc201903063 上一篇 下一篇
所属专题: 元素有机化学合辑2018-2019
综述与进展
收稿日期:2019-03-27
修回日期:2019-04-30
发布日期:2019-06-12
通讯作者:
陶雪芬
E-mail:34852349@qq.com
基金资助:
Tao Xuefena*( ), Sheng Rongb, Bao Kunb, Wang Yuxina, Jin Yinxiua
), Sheng Rongb, Bao Kunb, Wang Yuxina, Jin Yinxiua
Received:2019-03-27
Revised:2019-04-30
Published:2019-06-12
Contact:
Tao Xuefen
E-mail:34852349@qq.com
Supported by:文章分享
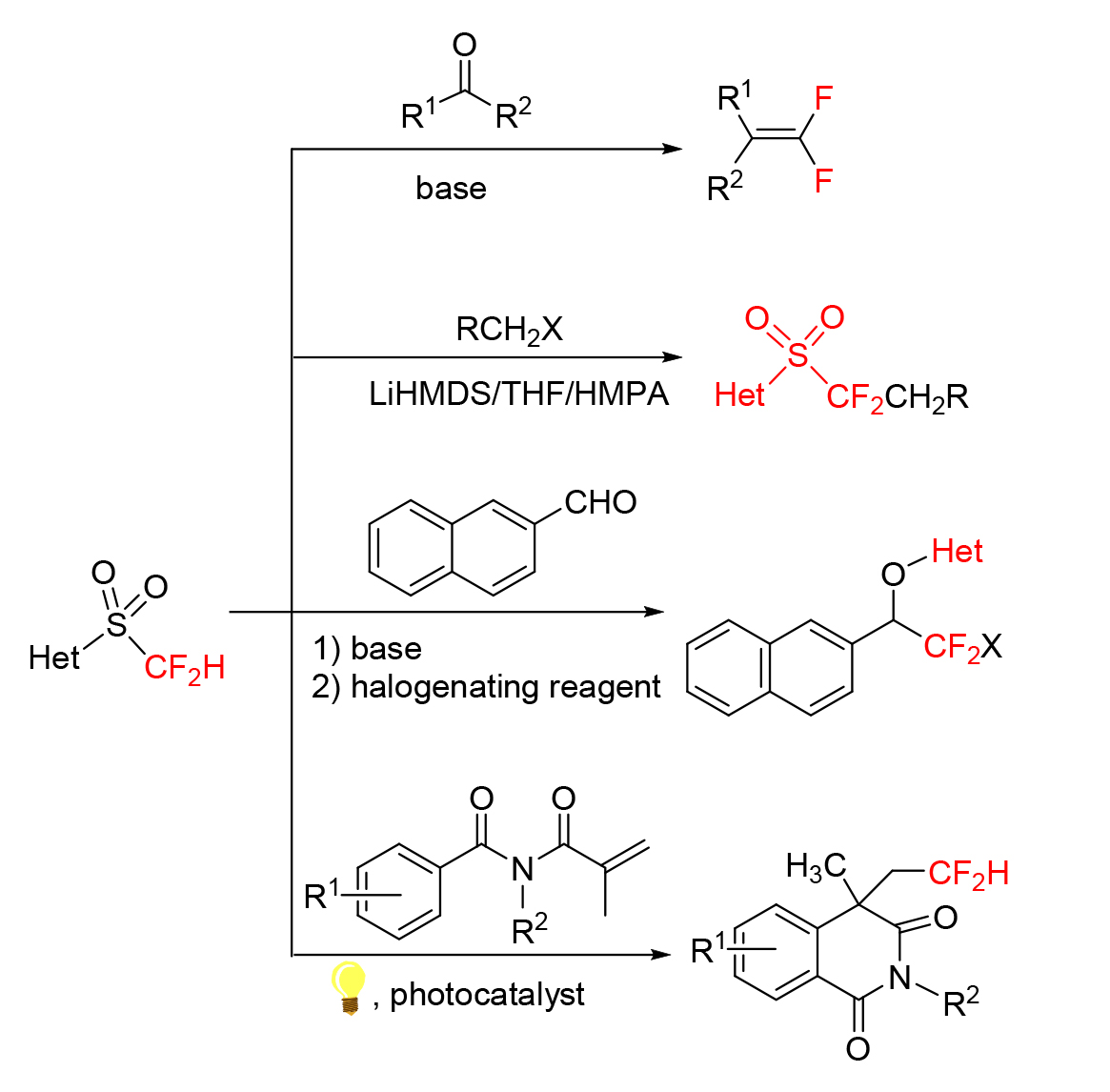
氟原子以及C—F键的独特性使得二氟亚甲基具有特殊的性质, 作为氧原子或羰基的生物电子等排体, 其在医药、农药与材料中起着有别于其他氟烷基的重要作用. 以二氟甲基2-吡啶基砜(胡试剂)为代表的二氟甲基杂芳基砜, 是近几年开发的新型二氟烷基化试剂, 因其具有易制备、官能团耐受性良好及对多种羰基化合物具有普遍适用性等优点而广受合成化学工作者的关注. 该类含氟试剂主要通过亲核取代反应、亲核加成反应、Julia-Kocienski烯化反应和自由基介导的双官能团化等反应, 将二氟甲基、二氟亚甲基、二氟烯基及其他二氟烷基引入醛、酮和杂环化合物的结构中. 首次从反应类型及其应用研究的角度综述了近十年来各种二氟甲基杂芳基砜参与的含氟有机化合物的合成研究.
陶雪芬, 盛荣, 鲍堃, 王玉新, 金银秀. 二氟甲基杂芳基砜作为二氟烷基化试剂的应用研究进展[J]. 有机化学, 2019, 39(10): 2726-2734.
Tao Xuefen, Sheng Rong, Bao Kun, Wang Yuxin, Jin Yinxiu. Progress of Difluoromethyl Heteroaryl Sulfones as Difluoroalkylation Reagents[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2726-2734.
| [1] |
Kanda, E . Bull. Chem. Soc. Jpn. 1937, 12, 469.
doi: 10.1246/bcsj.12.469 |
| [2] |
Premchandran, R. H.; Ogletree, M. L.; Fried, J . J. Org. Chem. 1993, 58, 5724.
doi: 10.1021/jo00073a035 |
| [3] |
Huchet, Q. A.; Kuhn, B.; Wagner, B.; Fischer, H.; Kansy, M.; Zimmerli, D.; Carreira, E. M.; Müller, K. J. Fluorine Chem. 2013, 152, 119.
doi: 10.1016/j.jfluchem.2013.02.023 |
| [4] | Liao, B.; Liao, Q. J. Prog. Pharm. Sci. 2012, 36, 138.(in Chinese). |
| ( 廖斌, 廖清江, 药学进展, 2012, 36, 138.) | |
| [5] | Tan, C. B.; Shi, L. L.; Wang, S. W. Drugs Clin. 2013, 28, 415 (in Chinese). |
| ( 谭初兵, 时丽丽, 王士伟 , 现代药物与临床, 2013, 28, 415.) | |
| [6] | Chen, L.; Zhao, T. X.; Zou, X. Chin. J. New Drug 2015, 24, 361(in Chinese). |
| ( 陈玲, 赵天笑, 邹栩 , 中国新药杂志, 2015, 24, 361.) | |
| [7] | Zhang, J. Z. Shanghai Med. Pharm. J. 2015, 36, 79(in Chinese). |
| ( 张建忠, 上海医药, 2015, 36, 79.) | |
| [8] |
Xiao, Y. L.; Zhang, B.; Feng, Z.; Zhang, X. G. Org. Lett. 2014, 16, 4822.
doi: 10.1021/ol502121m |
| [9] |
Zhang, X. X.; Cao, S. Tetrahedron Lett. 2017, 58, 375.
doi: 10.1016/j.tetlet.2016.12.054 |
| [10] | Ni, C. F.; Hu, M. Y.; Hu, J. B. Chem. Revv. 2015, 115, 765. |
| [11] |
Zhou, Q. Q.; Zou, Y. Q.; Lu, L. Q.; Xiao, W. J. Angew. Chem., Int. Ed. 2019, 58, 1586.
doi: 10.1002/anie.201803102 |
| [12] |
Grushin, V. V. A. Chem. Res. 2010, 43, 160.
doi: 10.1021/ar9001763 |
| [13] |
Hine, J.; Porter, J. J. J. Am. Chem. Soc. 1960, 82, 6178.
doi: 10.1021/ja01508a050 |
| [14] |
Zhao, Y. C.; Huang, W. Z.; Zhu, L. G.; Hu, J. B. Org. Lett. 2010, 12, 1444.
doi: 10.1021/ol100090r |
| [15] |
Zhao, Y. C.; Gao, B.; Hu, J. B. J. Am. Chem. Soc. 2012, 134, 5790.
doi: 10.1021/ja301601b |
| [16] |
Li, S.; Peng, P.; Wei, J.; Hu, Y. Z.; Hu, J. B.; Sheng, R . Adv. Synth. Catal. 2015, 357, 3429.
doi: 10.1002/adsc.201500150 |
| [17] | Habib, S.; Gueyrard, D . Eur. J. Org. Chem. 2015,871. |
| [18] | Liu, X.; Yin, Q.; Yin, J.; Chen, G. H.; Wang, X.; You, Q. D.; Chen, Y. L.; Xiong, B.; Shen, J. K . Eur. J. Org. Chem. 2014,6150. |
| [19] |
Moschitto, M. J.; Silverman, R. B . Org. Lett. 2018, 20, 4589.
doi: 10.1021/acs.orglett.8b01872 |
| [20] | Wang, X. P.; Lin, J. H.; Xiao, J. C.; Zheng, X . Eur. J. Org. Chem. 2014, 928. |
| [21] |
Gao, B.; Zhao, Y. C.; Hu, M. Y.; Ni, C. F.; Hu, J. B. Chem. Eur. J. 2014, 20, 1.
doi: 10.1002/chem.201390210 |
| [22] |
McAlpine, I.; Tran-Dubé, M.; Wang, F.; Scales, S.; Matthews, J.; Collins, M. R.; Nair, S. K.; Nguyen, M.; Bian, J. W.; Alsina, L. M.; Sun, J.; Zhong, J. Y.; Warmus, J. S.; O’Neill, B. T. J. Org. Chem. 2015, 80, 7266.
doi: 10.1021/acs.joc.5b00853 |
| [23] | Zhao, Y. C.; Zhang, L. J.; Xu, G. F.; Zheng, J.; Hu, J. B. Sci. China Chem. 2011, 41, 1833 (in Chinese). |
| ( 赵延川, 张丽君, 许国峰, 郑吉, 胡金波 , 中国科学•化学, 2011, 41, 1833.) | |
| [24] |
Prakash, G. K. S.; Ni, C. F.; Wang, F.; Zhang, Z.; Haiges, R Olah, G. A .; Angew. Chem., Int. Ed. 2013, 52, 10835.
doi: 10.1002/anie.201304395 |
| [25] |
Miao, W. J.; Ni, C. F.; Zhao, Y. C.; Hu, J. B. Org. Lett. 2016, 18, 2766.
doi: 10.1021/acs.orglett.6b01258 |
| [26] |
Prakash, G. K. S.; Ni, C. F.; Wang, F.; Hu, J. B.; George, A. O. Angew. Chem., Int. Ed. 2011, 50, 2559.
doi: 10.1002/anie.201007594 |
| [27] |
Zhou, Q.; Gui, J.; Pan, C. M.; Albone, E.; Cheng, X.; Suh, E. M.; Grasso, L.; Ishihara, Y.; Baran, P. S . J. Am. Chem. Soc. 2013, 135, 12994.
doi: 10.1021/ja407739y |
| [28] |
Zhou, Q.; Ruffoni, A.; Gianatassio, R.; Fujiwara, Y.; Sella, E.; Shabat, D.; Baran, P. S. Angew. Chem. 2013, 125, 4041.
doi: 10.1002/ange.v125.14 |
| [29] |
Gnaim, S.; Scomparin, A.; Li, X. L.; Baran, P. S.; Rader, C.; Ronit, S. F.; Shabat, D . Bioconjugate Chem. 2016, 27, 1965.
doi: 10.1021/acs.bioconjchem.6b00382 |
| [30] |
Pintauer, T.; Matyjaszewski, K. Chem. Soc. Rev. 2008, 37, 1087.
doi: 10.1039/b714578k |
| [31] |
Eckenhoff, W. T.; Pintauer, T. Catal. Rev. 2010, 52, 1.
doi: 10.1080/01614940903238759 |
| [32] |
Cao, M. Y.; Ren, X.; Lu, Z. Tetrahedron Lett. 2015, 56, 3732.
doi: 10.1016/j.tetlet.2015.04.091 |
| [33] |
Chen, J. R.; Yu, X. Y.; Xiao, W. J. Synthesis 2015, 47, 604.
doi: 10.1055/s-00000084 |
| [34] |
Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527.
doi: 10.1038/nchem.687 |
| [35] |
Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102.
doi: 10.1039/B913880N |
| [36] |
Xuan, J Xiao, W. J. .; Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1002/anie.201200223 |
| [37] |
Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322
doi: 10.1021/cr300503r |
| [38] | Koike, T.; Akita, M. Inorg. Chem. Front. 2014, 1, 561. |
| [39] |
Rong, J.; Deng, L.; Tan, P.; Ni, C. F.; Gu, Y. C.; Hu, J. B. Angew. Chem., Int. Ed. 2016, 55, 2743.
doi: 10.1002/anie.201510533 |
| [40] |
Zhu, M.; Fu, W. J.; Wang, Z. Q.; Xu, C.; Ji, B. M. Org. Biomol. Chem. 2017, 15, 9057.
doi: 10.1039/C7OB02366A |
| [41] |
Fu, W. J.; Han, X.; Zhu, M Xu, C.; Wang, Z. Q.; Ji, B. M.; Hao, X. Q.; Song, M .P. Chem. Commun. 2016, 52, 13413.
doi: 10.1039/C6CC07771D |
| [42] |
Zou, G. L.; Wang, X. L . Org. Biomol. Chem. 2017, 15, 8748.
doi: 10.1039/C7OB02226C |
| [43] |
He, Z. B.; Tan, P.; Ni, C. F.; Hu, J. B . Org. Lett. 2015, 17, 1838.
doi: 10.1021/acs.orglett.5b00308 |
| [44] |
Miao, W. J.; Zhao, Y. C.; Ni, C. F.; Gao, B.; Zhang, W.; Hu, J. B. J. Am. Chem. Soc. 2018, 140, 880.
doi: 10.1021/jacs.7b11976 |
| [45] | Yu, J. J.; Wu, Z.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 1. |
| [46] |
Miao, W. J.; Ni, C. F.; Zhao, Y. C.; Hu, J. B. J. Fluorine Chem. 2014, 167, 231.
doi: 10.1016/j.jfluchem.2014.05.012 |
| [47] |
Wei, J.; Gu, D. Y.; Wang, S. D.; Hu, J. B.; Dong, X. W.; Sheng, R. Org. Chem. Front. 2018, 5, 2568.
doi: 10.1039/C8QO00644J |
| [1] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [2] | 胡朝明, 吴纪红, 吴晶晶, 吴范宏. 直接三氟甲硒基化反应研究进展[J]. 有机化学, 2023, 43(1): 36-56. |
| [3] | 张广宇, 许家喜. 羟胺衍生物的[3,3] σ迁移反应及其应用[J]. 有机化学, 2021, 41(8): 3002-3014. |
| [4] | 袁文豪, 许家喜. 氧杂环丁烷的扩环反应[J]. 有机化学, 2021, 41(3): 947-958. |
| [5] | 崔大军,彭立军,曾宪顺,徐风波,张正之. 制备trans-Fe(CO)_3(PR_3)_2的新的取代过程[J]. 有机化学, 2003, 23(9): 973-976. |
| [6] | 阮继武,黄忠京,符立梧,马林,古练权. 胺类化合物与4,4’-二氟苯偶酰的亲核取代反应研究[J]. 有机化学, 2003, 23(8): 861-864. |
| [7] | 宣光荣,何宁德. 7,7-二氧双环[4,1,0]庚烷与活性亚甲基化合物的反应[J]. 有机化学, 2003, 23(7): 734-736. |
| [8] | 王建武,贾炯,候殿杰,李红梅,尹军. 一个新的制备咪唑并[1,5-α]吡啶衍生物的串联反应[J]. 有机化学, 2003, 23(2): 173-175. |
| [9] | 张素娜,于建新,李中军,孙万赋,周蓉,张丽静,王瑞英,蔡孟深. 6-S-(取代的三或四唑杂环基)-1,2:3,4-二-O-异亚丙基-α-D吡喃型半乳糖的合成研究[J]. 有机化学, 2003, 23(2): 176-181. |
| [10] | 王进军,殷军港,邬旭然,赵岩,森章,初井敏一,竹下齐. 萜品油烯的DeMayo光环加成产物的反应研究[J]. 有机化学, 2003, 23(10): 1120-1124. |
| [11] | 李伟章,恽榴红,王好山. 微波辐射促进N-烷基化邻硝基苯胺的合成[J]. 有机化学, 2002, 22(7): 511-514. |
| [12] | 胡方中,王翔,任康太,杨华铮. 芳氧基哒嗪氧基乳酸酯合成条件的研究--3,6-二卤代哒嗪中卤原子对其亲核取代反应的影响[J]. 有机化学, 2002, 22(6): 417-422. |
| [13] | 魏琦,麻生明. 1,2-联烯亚砚和1,2-联烯砜的反应[J]. 有机化学, 2002, 22(4): 254-261. |
| [14] | 戴秋云,黄启斌,邓云度. 一种高亲核怀长链咪唑肟的合成与反应[J]. 有机化学, 2002, 22(2): 130-134. |
| [15] | 王昊阳,郭寅龙,张亮,安登魁. 有机质谱学法研究气相S_N2离子-分子反应进展[J]. 有机化学, 2002, 22(12): 974-980. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||