有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3679-3694.DOI: 10.6023/cjoc202306020 上一篇 下一篇
综述与进展
收稿日期:2023-06-22
修回日期:2023-08-04
发布日期:2023-08-22
基金资助:
Zimeng Shao, Yuanhui Wang, Haiying Wang, Peipei Cui( ), Xuguang Liu(
), Xuguang Liu( )
)
Received:2023-06-22
Revised:2023-08-04
Published:2023-08-22
Contact:
E-mail: Supported by:文章分享

稠环芳烃具有独特的电子性质和光学性质, 在有机光电材料领域应用广泛. 向稠环芳烃骨架中引入杂原子可改变其电子结构, 进而改变其物理、化学、光、电和磁等性质. 在众多的杂原子中, 氮原子是稠环芳烃中最常见的掺杂原子. 呜拉嗪是芘的等电子体, 又称吲哚并[6,5,4,3-ija]喹啉, 是一种新型氮杂稠环芳烃. 近年来, 呜拉嗪在染料敏化太阳能电池等领域表现出了潜在应用, 随后有关呜拉嗪的合成方法研究备受关注. 总结了近年来呜拉嗪及其衍生物的主要合成方法.
邵梓萌, 王原辉, 王海滢, 崔培培, 刘旭光. 呜拉嗪及其衍生物的合成研究进展[J]. 有机化学, 2023, 43(11): 3679-3694.
Zimeng Shao, Yuanhui Wang, Haiying Wang, Peipei Cui, Xuguang Liu. Recent Advance in the Synthesis of Ullazine and Its Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3679-3694.
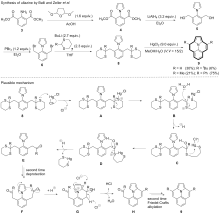

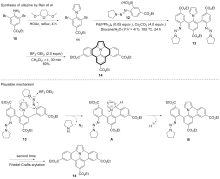

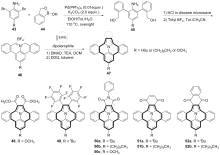
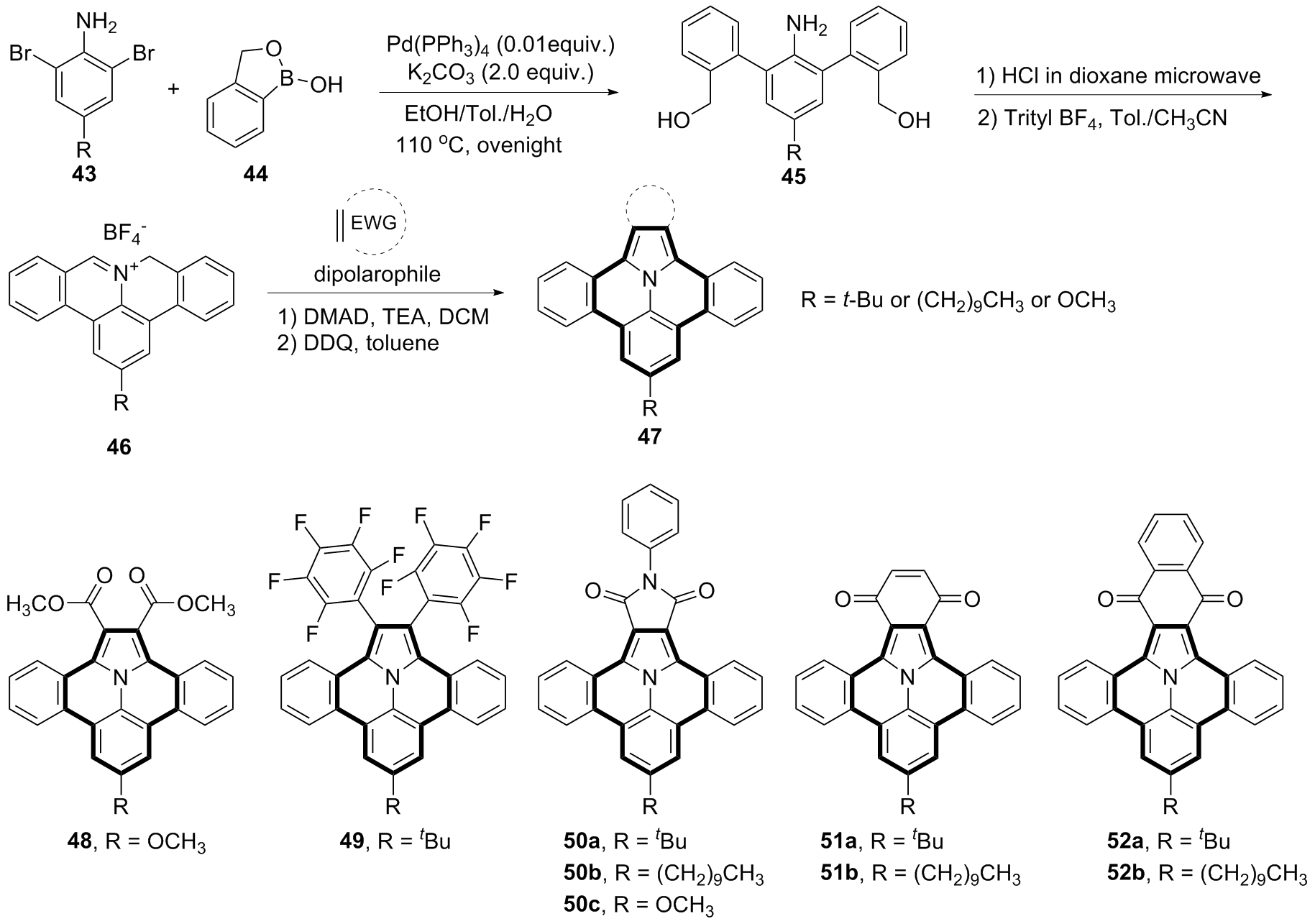
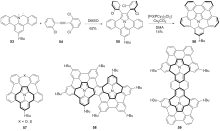

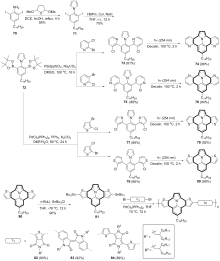

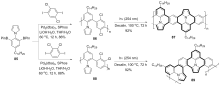

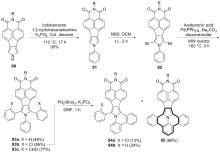
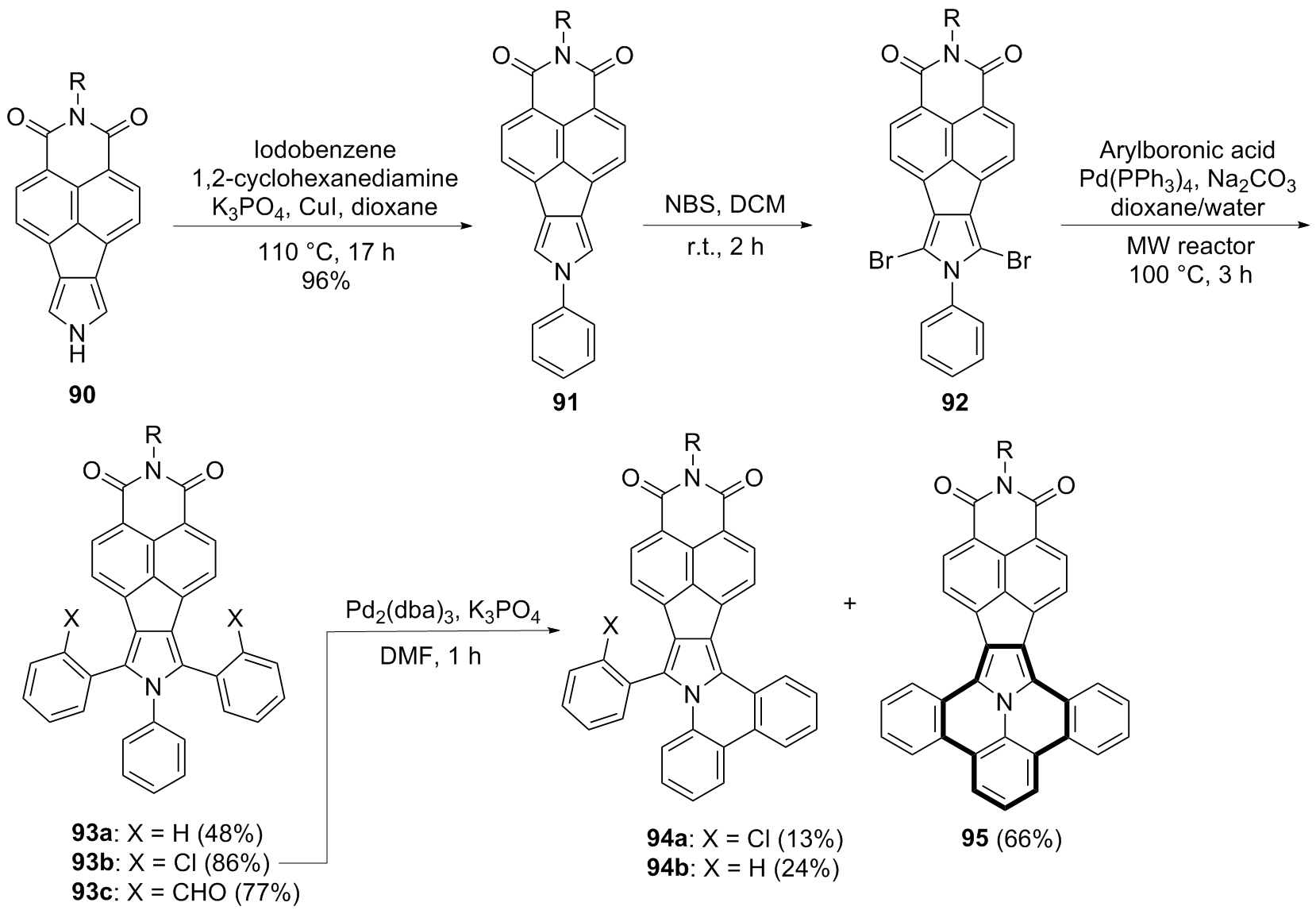
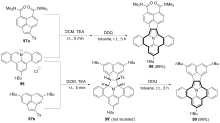


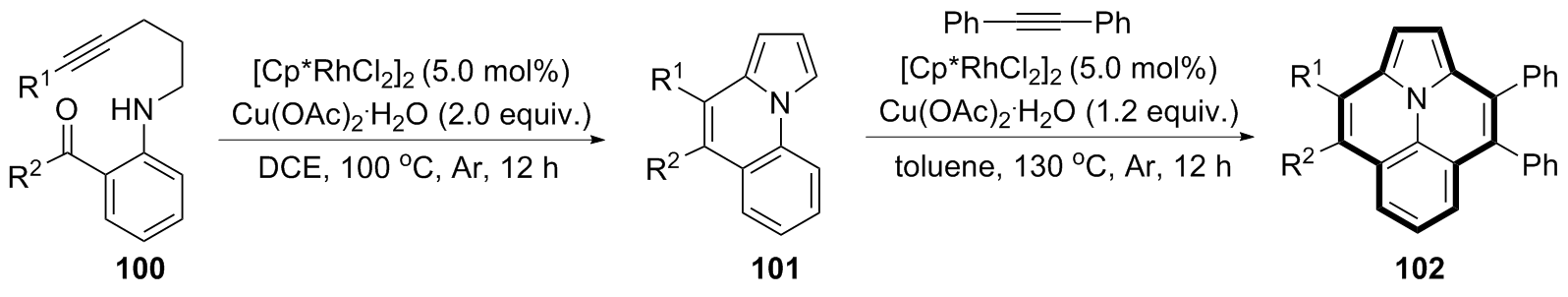
| [1] |
Cebrián, C. J. Mater. Chem. C 2018, 6(44), 11943.
doi: 10.1039/C8TC03573C |
| [2] |
Bunz, U. H. F. Acc. Chem. Res. 2015, 48(6), 1676.
doi: 10.1021/acs.accounts.5b00118 |
| [3] |
Takase, M.; Enkelmann, V.; Sebastiani, D.; Baumgarten, M.; Müllen, K. Angew. Chem. Int. Ed. 2007, 46(29), 5524.
doi: 10.1002/anie.v46:29 |
| [4] |
Balli, H.; Zeller, M. Helv. Chim. Acta. 1983, 66(7), 2135.
doi: 10.1002/hlca.v66:7 |
| [5] |
Zhou, J.; Yang, W. J.; Wang, B. J.; Ren, H. J. Angew. Chem. Int. Ed. 2012, 51(49), 12293.
doi: 10.1002/anie.201206578 pmid: 23124928 |
| [6] |
Kanno, K. I.; Liu, Y. H.; Iesato, A.; Nakajima, K.; Takahashi, T. Org. Lett. 2005, 7(24), 5453.
doi: 10.1021/ol052214x |
| [7] |
Delcamp, J. H.; Yella, A.; Holcombe, T.W.; Nazeeruddin, M. K.; Grätzel, M. Angew. Chem. Int. Ed. 2013, 52(1), 376.
doi: 10.1002/anie.201205007 pmid: 22927088 |
| [8] |
Nazeeruddin, M. K.; Drigo, N.; Sanghyun, P.; Huckaba, A.; Schouwink, P.; Tabet, N. Chem.-Eur. J. 2017, 23(68), 17209.
doi: 10.1002/chem.v23.68 |
| [9] |
Zhang, Y. B.; Cheema, H.; McNamara, L.; Hunt, L. A.; Hammer, N.; Delcamp, J. H. Chem.-Eur. J. 2018, 24(22), 5939.
doi: 10.1002/chem.v24.22 |
| [10] |
Wan, D. Y.; Li, X. Y.; Jiang, R. Y.; Feng, B. Y.; Lan, J. B.; Wang, R. L.; You, J. S. Org. Lett. 2016, 18(12), 2876.
doi: 10.1021/acs.orglett.6b01182 |
| [11] |
Larock, R. C.; Doty, M. J.; Han, X.J. Tetrahedron Lett. 1998, 39(29), 5143.
doi: 10.1016/S0040-4039(98)00982-4 |
| [12] |
Das, A.; Ghosh, I.; König, B. Chem. Commun. 2016, 52(56), 8695.
doi: 10.1039/C6CC04366F |
| [13] |
Wang, D. P.; Liu, Y.; Wang, L. H.; Cheng, H.; Zhang, Y. M.; Gao, G. Chin. Chem. Lett. 2020, 32(4), 1407.
doi: 10.1016/j.cclet.2020.09.057 |
| [14] |
Berger, R.; Wagner, M.; Feng, X. L.; Müllen, K. Chem. Sci. 2015, 6(1), 436.
doi: 10.1039/C4SC02793K |
| [15] |
Berger, R.; Giannakopoulos, A.; Ravat, P.; Wagner, M.; Beljonne, D.; Feng, X. L.; Müllen, K. Angew. Chem. Int. Ed. 2014, 53(39), 10520.
doi: 10.1002/anie.v53.39 |
| [16] |
Ito, S.; Tokimaru, Y.; Nozaki, K. Chem. Commun. 2015, 54(259), 7256.
doi: 10.1039/C8CC90288G |
| [17] |
Wang, W. F.; Hanindita, F.; Tanaka, Y.; Ochiai, K.; Sato, H.; Li, Y. X.; Yasuda, T.; Ito, S. Angew. Chem. Int. Ed. 2023, 62(8), e202218176.
doi: 10.1002/anie.v62.8 |
| [18] |
Wang, W. F.; Hanindita, F.; Webster, R. D.; Ito, S. CCS Chem. 2022, 1.
|
| [19] |
Wang, W. F.; Hanindita, F.; Hamamoto, Y.; Li, Y. X.; Ito, S. Nat. Commun. 2022, 13(1), 1498.
doi: 10.1038/s41467-022-29106-w |
| [20] |
Guo, X. Y.; Yuan, Z. Y.; Zhu, Y. P.; Li, Z. H.; Huang, R. K.; Xia, Z. M.; Zhang, W. X.; Li, Y.; Wang, J. B. Angew. Chem. Int. Ed. 2019, 58(47), 16966.
doi: 10.1002/anie.v58.47 |
| [21] |
Miao, D.; Aumaitre, C.; Morin, J-F. J. Mater. Chem. C 2019, 7(10), 3015.
doi: 10.1039/C8TC05288C |
| [22] |
Miao, D.; Michele, V. D.; Gagnon, F.; Aumaître, C.; Lucotti, A.; Zoppo, M. D.; Lirette, F.; Tommasini, M.; Morin, J-F. J. Am. Chem. Soc. 2021, 143(30), 11302.
doi: 10.1021/jacs.1c05616 |
| [23] |
Hager, J.; Kang, S.; Chmielewski, P. J.; Lis, T.; Kim, D.; Stępień, M. Org. Chem. Front. 2022, 9(12), 3179.
doi: 10.1039/D2QO00421F |
| [24] |
Hu, Y. C.; Jia, Y. Y.; Tuo, Z. K.; Zhou, W. Org. Lett. 2023, 25(11), 1845.
doi: 10.1021/acs.orglett.3c00321 |
| [25] |
Hou, D. F.; Balli, H. Helv. Chim. Acta 1992, 75(8), 2608.
doi: 10.1002/hlca.v75:8 |
| [26] |
(a) Rubio-Presa, R.; Pedrosa, M. R.; Fernández-Rodríguez, M. A.; Arnáiz, F. J.; Sanz, R. Org. Lett. 2017, 19(19), 5470.
doi: 10.1021/acs.orglett.7b02792 pmid: 28952319 |
|
(b) Hernández-Ruiz, R.; Rubio-Presa, R.; Suárez-Pantiga, S.; Pedrosa, M. R.; Fernández-Rodríguez, M. A.; Tapia, M. J.; Sanz, R. Chem. Eur. J. 2021, 27(54), 13613.
doi: 10.1002/chem.v27.54 pmid: 28952319 |
|
| [27] |
Boldt, S.; Parpart, S.; Villinger, A.; Ehlers, P.; Langer P. Chem. Int. Ed. 2017, 56(16), 4575.
doi: 10.1002/anie.v56.16 |
| [28] |
Pierrat, P.; Hesse, S.; Cebrián, C.; Gros, P. C. Org. Biomol. Chem. 2017, 15(40), 8568.
doi: 10.1039/C7OB02149F |
| [29] |
Parpart, S.; Boldt, S.; Ehlers, P.; Langer, P. Org. Lett. 2018, 20(1), 122.
doi: 10.1021/acs.orglett.7b03477 pmid: 29232149 |
| [30] |
Ibrahim, D.; Boulet, P.; Gros, P. C.; Pierrat, P. Eur. J. Org. Chem. 2021, 2021(22), 3331.
doi: 10.1002/ejoc.v2021.22 |
| [31] |
Ge, Q. M.; Li, B.; Wang, B. Q. Org. Biomol. Chem. 2016, 14(5), 1814.
doi: 10.1039/C5OB02515J |
| [32] |
Li, Q-Q.; Ochiai, K.; Lee, C-A.; Ito, S. Org. Lett. 2020, 22(15), 6132.
doi: 10.1021/acs.orglett.0c02203 |
| [33] |
Ghorai, D.; Choudhury, J. ACS Catal. 2015, 5(4), 2692.
doi: 10.1021/acscatal.5b00243 |
| [34] |
Li, Q-Q.; Hamamoto, Y.; Tan, C. C. H.; Sato, H.; Ito, S. Org. Chem. Front. 2022, 9(15), 4128.
doi: 10.1039/D2QO00941B |
| [35] |
Davies, D. L.; Ellul, C. E.; Macgregor, S. A.; McMullin, C. L.; Singh, K. J. Am. Chem. Soc. 2015, 137(30), 9659.
doi: 10.1021/jacs.5b04858 pmid: 26115418 |
| [36] |
Villar, J. M.; Suárez, J.; Varela, J. A.; Saá, C. Org. Lett. 2017, 19(7), 1702.
doi: 10.1021/acs.orglett.7b00478 pmid: 28301167 |
| [37] |
Wang, T. B.; Zheng, Q. Z.; Wang, L. H.; Huang, Z. M.; Zhang, H. X.; Zhang, Y. M.; Zhang, C.; Gao, G. Chem. Commun. 2022, 58(4), 541.
doi: 10.1039/D1CC06194A |
| [38] |
(a) Mellerup, S. K.; Wang, S. N. Trends Chem. 2019, 1(1), 77.
doi: 10.1016/j.trechm.2019.01.003 |
|
(b) Sun, W. T.; Guo, J. X.; Fan, Z. M.; Yuan, L. Z.; Ye, K. Q.; Dou, C. D.; Wang, Y. Angew. Chem. Int. Ed. 2022, 61(40), e202209271.
doi: 10.1002/anie.v61.40 |
|
|
(c) Zhao, R. Y.; Liu, J.; Wang, L. X. Acc. Chem. Res. 2020, 53(8), 1557.
doi: 10.1021/acs.accounts.0c00281 |
|
| [39] |
(a) Xu, X. Y.; Liu, M. Y.; Li, C. L.; Liu, X. G. Chin. J. Org. Chem. 2023, 43(5), 1611.
doi: 10.6023/cjoc202212038 |
|
(b) Wang, J-Y.; Pei, J. Chin. Chem. Lett. 2016, 27(8), 1139.
doi: 10.1016/j.cclet.2016.06.014 |
|
|
(c) Campbell, P. G.; Marwitz, A. J. V.; Liu, S-Y. Angew. Chem. Int. Ed. 2012, 51(18), 6074.
doi: 10.1002/anie.v51.25 |
|
| [40] |
Li, C. L.; Liu, Y. M.; Sun, Z.; Zhang, J. Y.; Liu, M. Y.; Zhang, C.; Zhang, Q.; Wang, H. J.; Liu, X. G. Org. Lett. 2018, 20(10), 2806.
doi: 10.1021/acs.orglett.8b00554 |
| [41] |
Guo, Y. K.; Zhang, L.; Li, C. L.; Jin, M. J.; Zhang, Y. L.; Ye, J. C.; Chen, Y.; Wu, X. M.; Liu, X. G. J. Org. Chem. 2021, 86(18), 12507.
doi: 10.1021/acs.joc.1c00777 |
| [1] | 张祎, 杜呈卓, 李继坤, 王小野. 基于硼氮杂稠环芳烃的多重共振热活化延迟荧光材料研究进展[J]. 有机化学, 2023, 43(5): 1645-1690. |
| [2] | 徐晓阳, 刘美艳, 李成龙, 刘旭光. 1,2-硼氮杂芳烃在中国的研究进展[J]. 有机化学, 2023, 43(5): 1611-1644. |
| [3] | 徐晓阳, 刘美艳, 李成龙, 吴晓明, 刘旭光. 1,4-硼氮杂芳烃在中国的研究进展[J]. 有机化学, 2023, 43(11): 3826-3843. |
| [4] | 尹艳丽, 赵筱薇, 江智勇. 可见光不对称催化合成手性氮杂芳烃衍生物[J]. 有机化学, 2022, 42(6): 1609-1625. |
| [5] | 徐东平, 黄飞, 汤琳, 张新明, 张武. 可见光诱导杂芳烃与脂肪醇的羟烷基化反应[J]. 有机化学, 2022, 42(5): 1493-1500. |
| [6] | 丁邦东, 姜业朝, 张瑜, 叶蓉, 孙晶, 颜朝国. 喹啉季铵盐和1,3-茚满二酮及2-芳亚甲基1,3-茚满二酮的环加成反应合成茚满酮类含氮杂环化合物[J]. 有机化学, 2020, 40(4): 1003-1016. |
| [7] | 潘世豪, 缪谦. 利用扩环反应策略向稠环芳烃引入八元环[J]. 有机化学, 2020, 40(10): 3347-3353. |
| [8] | 艾锋, 谢宗波, 姜国芳, 季久健, 乐长高. 离子液体水溶液中2-甲基氮杂芳烃苄位C(sp3)-H官能化反应[J]. 有机化学, 2018, 38(8): 2174-2181. |
| [9] | 肖尚友, 毕旌富, 穆小静, 周曌, 徐广. 高温水中2-甲基喹啉及2-甲基吡啶与芳醛的反应研究[J]. 有机化学, 2017, 37(8): 2159-2164. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||