有机化学 ›› 2024, Vol. 44 ›› Issue (5): 1385-1402.DOI: 10.6023/cjoc202312014 上一篇 下一篇
综述与进展
收稿日期:2023-12-15
修回日期:2024-01-13
发布日期:2024-01-30
基金资助:Received:2023-12-15
Revised:2024-01-13
Published:2024-01-30
Contact:
*E-mail: Supported by:文章分享
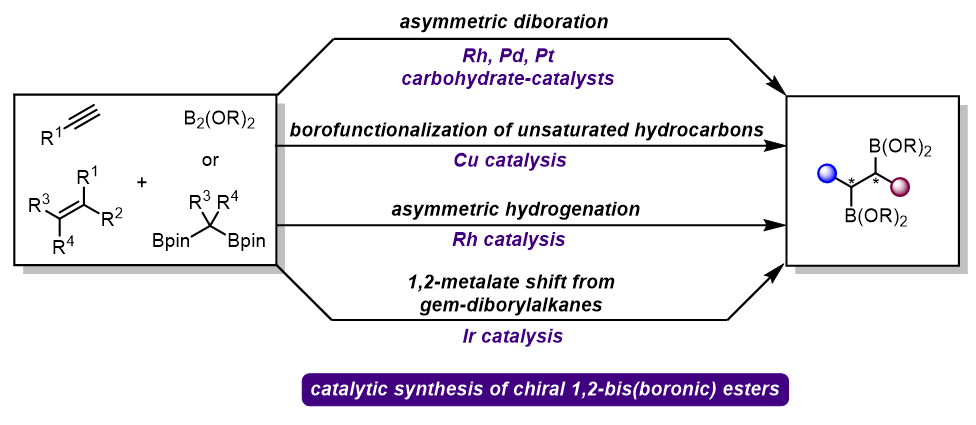
手性1,2-双硼酸酯是合成化学中重要的转化砌块, 其催化不对称合成引起了化学界的广泛关注. 近年来, 过渡金属和手性双醇催化的烯烃不对称双硼化反应已成为合成手性1,2-双硼酸酯的重要方法, 基于烯基双硼化合物的不对称氢化反应, 可以作为烯烃双硼化反应的补充来合成对应的目标产物. 同时, 烯烃或炔烃的硼化/官能团化也是构建这类化合物的另一种有效方法. 最近, 以偕二硼酸酯为起始物的催化不对称迁移增碳反应, 为手性1,2-双硼酸酯的合成提供了新的思路. 总结了手性1,2-双硼酸酯合成的最新研究进展及面临的挑战, 并对未来的研究方向进行了展望.
吉崇磊, 高得伟. 不对称催化合成手性1,2-双硼酸酯研究进展[J]. 有机化学, 2024, 44(5): 1385-1402.
Chonglei Ji, Dewei Gao. Recent Advances in Catalytic Asymmetric Synthesis of Chiral 1,2-Bis(boronic) Esters[J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1385-1402.
| [1] |
(a) Caldwell, J. J. Clin. Pharmacol. 1992, 32, 925.
pmid: 1447400 |
|
(b) Jozwiak, K.; Lough, W. J.; Wainer, I. W. Drug Stereochemistry: Analytical Methods and Pharmacology, 3rd ed., Informa, New York, 2012.
pmid: 1447400 |
|
|
(c) Rentsch, K. M. J. Biochem. Biophys. Methods 2002, 54, 1.
pmid: 1447400 |
|
| [2] |
(a) Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed., Wiley-VCH, Weinheim, Germany, 2011.
pmid: 32612487 |
|
(b) Trippier, P. C.; McGuigan, C. Med. Chem. Commun. 2010, 1, 183.
pmid: 32612487 |
|
|
(c) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
pmid: 32612487 |
|
|
(d) Suzuki, A. Angew. Chem., Int. Ed. 2011, 50, 6722.
doi: 10.1002/anie.201101379 pmid: 32612487 |
|
|
(e) Xu, L.; Zhang, S.; Li, P. Chem. Soc. Rev. 2015, 44, 8848.
pmid: 32612487 |
|
|
(f) Brooks, W. L. A.; Sumerlin, B. S. Chem. Rev. 2016, 116, 1375.
pmid: 32612487 |
|
|
(g) Diner, C.; Szabó, K. J. J. Am. Chem. Soc. 2017, 139, 2.
pmid: 32612487 |
|
|
(h) Fyfe, J. W. B.; Watson, A. J. B. Chem 2017, 3, 31.
pmid: 32612487 |
|
|
(i) Rygus, J. P. G.; Crudden, C. M. J. Am. Chem. Soc. 2017, 139, 18124.
pmid: 32612487 |
|
|
(j) Namirembe, S.; Morken, J. P. Chem. Soc. Rev. 2019, 48, 3464.
doi: 10.1039/c9cs00180h pmid: 32612487 |
|
|
(k) He, Z.; Hu, Y.; Xia, C.; Liu, C. Org. Biomol. Chem. 2019, 17, 6099.
pmid: 32612487 |
|
|
(l) Kischkewitz, M.; Friese, F. W.; Studer, A. Adv. Synth. Catal. 2020, 362, 2077.
doi: 10.1002/adsc.201901503 pmid: 32612487 |
|
|
(m) Kalita, S. J.; Cheng, F.; Huang, Y.-Y. Adv. Synth. Catal. 2020, 362, 2778.
pmid: 32612487 |
|
|
(n) Yang, K.; Song, Q. Acc. Chem. Res. 2021, 54, 2298.
pmid: 32612487 |
|
|
(o) Yeung, K.; Mykura, R. C.; Aggarwal, V. K. Nat. Synth. 2022, 1, 117.
pmid: 32612487 |
|
|
(p) Jiang, X.-M.; Liu, X.-R.; Chen, A.; Zou, X.-Z.; Ge, J.-F.; Gao, D.-W. Eur. J. Org. Chem. 2022, e202101463.
pmid: 32612487 |
|
| [3] |
(a) Viso, A.; Fernández de la Pradilla, R.; Tortosa, M. ACS Catal. 2022, 12, 10603.
|
|
(b) Wang, X.; Wang, Y.; Huang, W.; Xia, C.; Wu, L. ACS Catal. 2021, 11, 1.
|
|
| [4] |
(a) Mlynarski, S. N.; Schuster, C. H.; Morken, J. P. Nature 2014, 505, 386.
pmid: 33180502 |
|
(b) Blaisdell, T. P.; Morken, J. P. J. Am. Chem. Soc. 2015, 137, 8712.
doi: 10.1021/jacs.5b05477 pmid: 33180502 |
|
|
(c) Crudden, C. M.; Ziebenhaus, C.; Rygus, J. P. G.; Ghozati, K.; Unsworth, P. J.; Nambo, M.; Voth, S.; Hutchinson, M.; Laberge, V. S.; Maekawa, Y.; Imao, D. Nat. Commun. 2016, 7, 11065.
pmid: 33180502 |
|
|
(d) Fawcett, A.; Nitsch, D.; Ali, M.; Bateman, J. M.; Myers, E. L.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2016, 55, 14663.
pmid: 33180502 |
|
|
(e) Liu, X.; Sun, C.; Mlynarski, S.; Morken, J. P. Org. Lett. 2018, 20, 1898.
pmid: 33180502 |
|
|
(f) Davenport, E.; Fernandez, E. Chem. Commun. 2018, 54, 10104.
pmid: 33180502 |
|
|
(g) Fawcett, A.; Murtaza, A.; Gregson, C. H. U.; Aggarwal, V. K. J. Am. Chem. Soc. 2019, 141, 4573.
pmid: 33180502 |
|
|
(h) Namirembe, S.; Yan, L.; Morken, J. P. Org. Lett. 2020, 22, 9174.
doi: 10.1021/acs.orglett.0c03134 pmid: 33180502 |
|
|
(i) Willems, S.; Toupalas, G.; Reisenbauer, J. C.; Morandi, B. A. Chem. Commun. 2021, 57, 3909.
pmid: 33180502 |
|
|
(j) Mali, M.; Sharma, G. V. M.; Ghosh, S.; Roisnel, T.; Carboni, B.; Berrée, F. J. Org. Chem. 2022, 87, 7649.
pmid: 33180502 |
|
|
(k) Xu, N.; Kong, Z.; Wang, J. Z.; Lovinger, G. J.; Morken, J. P. J. Am. Chem. Soc. 2022, 144, 17815.
pmid: 33180502 |
|
|
(l) Zhang, M.; Lee, P. S.; Allais, C.; Singer, R. A.; Morken, J. P. J. Am. Chem. Soc. 2023, 145, 8308.
pmid: 33180502 |
|
| [5] |
Ma, X.; Murray, B.; Biscoe, M. R. Nat. Rev. Chem. 2020, 4, 584.
|
| [6] |
Blair, D. J.; Tanini, D.; Bateman, J. M.; Scott, H. K.; Myers, E. L.; Aggarwal, V. K. Chem. Sci. 2017, 8, 2898.
|
| [7] |
(a) Coombs. J. R.; Morken. J. P. Angew. Chem., Int. Ed. 2016, 55, 2636.
doi: 10.1002/anie.201507151 pmid: 30740530 |
|
(b) Obligacion, J. V.; Chirik, P. J. Nat. Rev. Chem. 2018, 2, 15.
doi: 10.1038/s41570-018-0001-2 pmid: 30740530 |
|
|
(c) Wang, F.; Chen, P.; Liu, G. Acc. Chem. Res. 2018, 51, 2036.
pmid: 30740530 |
|
|
(d) Chen, J.-H.; Guo. J.; Lu, Z. Chin. J. Chem. 2018, 36, 1075.
pmid: 30740530 |
|
|
(e) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Chem. Soc. Rev. 2020, 49, 32.
pmid: 30740530 |
|
| [8] |
Morgan, J. B.; Miller, S. P.; Morken, J. P. J. Am. Chem. Soc. 2003, 125, 8702.
|
| [9] |
Miller, S. P.; Morgan, J. B.; Nepveux, V. F. J.; Morken, J. P. Org. Lett. 2004, 6, 131.
|
| [10] |
Trudeau, S.; Morgan, J. B.; Shrestha, M.; Morken, J. P. J. Org. Chem. 2005, 70, 9538.
|
| [11] |
Toribatake, K.; Nishiyama, H. Angew. Chem., Int. Ed. 2013, 52, 11011.
doi: 10.1002/anie.201305181 pmid: 24000239 |
| [12] |
Pelz, N. F.; Woodward, A. R.; Burks, H. E.; Sieber, J. D.; Morken, J. P. J. Am. Chem. Soc. 2004, 126, 16328.
|
| [13] |
Sieber, J. D.; Morken, J. P. J. Am. Chem. Soc. 2006, 128, 74.
|
| [14] |
Woodward, A. R.; Burks, H. E.; Chan, L. M.; Morken, J. P. Org. Lett. 2005, 7, 5505.
pmid: 16288542 |
| [15] |
Pelz, N. F.; Morken, J. P. Org. Lett. 2006, 8, 4557.
|
| [16] |
Kliman, L. T.; Mlynarski, S. N.; Ferris, G. E.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 13210.
doi: 10.1021/ja9047762 pmid: 19702329 |
| [17] |
(a) Kliman, L. T.; Mlynarski, S. N.; Morken, J. P. Angew. Chem., Int. Ed. 2012, 51, 512.
pmid: 23862690 |
|
(b) Coombs, J. R.; Haeffner, F.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2013, 135, 11222.
doi: 10.1021/ja4041016 pmid: 23862690 |
|
| [18] |
Ferris, G. E.; Hong, K.; Roundtree, I. A.; Morken, J. P. J. Am. Chem. Soc. 2013, 135, 2501.
doi: 10.1021/ja400506j pmid: 23390951 |
| [19] |
Coombs, J. R.; Zhang, L.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 16140.
doi: 10.1021/ja510081r pmid: 25387002 |
| [20] |
(a) Byrom, N. T.; Grigg, R.; Kongkathip, B.; Reimer, G.; Wade, A. R. J. Chem. Soc., Perkin Trans. 1 1984, 1643.
|
|
(b) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. Tetrahedron Lett. 1986, 27, 3535.
|
|
|
(c) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. J. Chem. Soc., Chem. Commun. 1988, 356.
|
|
|
(d) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. J. Chem. Soc., Perkin Trans. 1 1990, 1375.
|
|
|
(e) Mayer, S. F.; Mang, H.; Steinreiber, A.; Saf, R.; Faber, K. Can. J. Chem. 2002, 80, 362.
|
|
| [21] |
Nóvoa, L.; Trulli, L.; Parra, A.; Tortosa, M. Angew. Chem., Int. Ed. 2021, 60, 11763.
doi: 10.1002/anie.202101445 pmid: 33689223 |
| [22] |
(a) Bonet, A.; Sole, C.; Gulyás, H.; Fernández, E. Org. Biomol. Chem. 2012, 10, 6621.
|
|
(b) Bonet, A.; Pubill-Ulldemolins, C.; Bo, C.; Gulyás, H.; Fernández, E. Angew. Chem., Int. Ed. 2011, 50, 7158.
|
|
| [23] |
Fang, L.; Yan, L.; Haeffner, F.; Morken, J. P. J. Am. Chem. Soc. 2016, 138, 2508.
|
| [24] |
Yan, L.; Meng, Y.; Haeffner, F.; Leon, R. M.; Crockett, M. P.; Morken, J. P. J. Am. Chem. Soc. 2018, 140, 3663.
doi: 10.1021/jacs.7b12316 pmid: 29442502 |
| [25] |
Yan, L.; Morken, J. P. Org. Lett. 2019, 21, 3760.
doi: 10.1021/acs.orglett.9b01204 pmid: 31066564 |
| [26] |
Lee, Y.; Jang, H.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 18234.
|
| [27] |
Lee, Y.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 3160.
|
| [28] |
Jung, H.-Y.; Yun, J. Org. Lett. 2012, 14, 2606.
|
| [29] |
Zanghi, J. M.; Liu, S.; Meek, S. J. Org. Lett. 2019, 21, 5172.
|
| [30] |
Radomkit, S.; Liu, Z.; Closs, A.; Mikus, M. S.; Hoveyda, A. H. Tetrahedron 2017, 73, 5011.
|
| [31] |
Lee, H.; Lee, S.; Yun, J. ACS Catal. 2020, 10, 2069.
|
| [32] |
Green, J. C.; Joannou, M. V.; Murray, S. A.; Zanghi, J. M.; Meek, S. J. ACS Catal. 2017, 7, 4441.
|
| [33] |
Fan, Z.; Ye, M.; Wang, Y.; Qiu, J.; Li, W.; Ma, X.; Yang, K.; Song, Q. ACS Cent. Sci. 2022, 8, 1134.
|
| [34] |
Morgan, J. B.; Morken, J. P. J. Am. Chem. Soc. 2004, 126, 15338.
|
| [35] |
Paptchikhine, A.; Cheruku, P.; Engman, M.; Andersson, P. G. Chem. Commun. 2009, 5996.
|
| [36] |
(a) Allen, A. E.; MacMillan, D. W. C. Chem. Sci. 2012, 3, 633.
|
|
(b) Pye, D. R.; Mankad, N. P. Chem. Sci. 2017, 8, 1705.
|
|
|
(c) Fu, J.; Huo, X.; Li, B.; Zhang, W. Org. Biomol. Chem. 2017, 15, 9747.
|
|
|
(d) Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S.-g. Chem. Rev. 2020, 120, 13382.
|
|
|
(e) Tian, F.; Zhang, J.; Yang, W.-L.; Deng, W.-P. Chin. J. Org. Chem. 2020, 40, 3262.
|
|
|
(f) Huo, X.; Li, G.; Wang, X.; Zhang, W. Angew. Chem., Int. Ed. 2022, 61, e202210086.
|
|
|
(g) Wei, L.; Wang, C.-J. Chin. J. Chem. 2021, 39, 15.
|
|
|
(h) Martínez, S.; Veth, L.; Lainer, B.; Dydio, P. ACS Catal. 2021, 11, 3891.
|
|
|
(i) Wei, L.; Wang, C.-J. Chem. Catal. 2023, 3, 100455.
|
|
| [37] |
(a) Wang, Y.; Liu, X.; Deng, L. J. Am. Chem. Soc. 2006, 128, 3928.
pmid: 26662073 |
|
(b) Wang, B.; Wu, F.; Wang, Y.; Liu, X.; Deng, L. J. Am. Chem. Soc. 2007, 129, 768.
pmid: 26662073 |
|
|
(c) Zhu, B.; Lee, R.; Li, J.; Ye, X.; Hong, S.-N.; Qiu, S.; Coote, M. L.; Jiang, Z. Angew. Chem., Int. Ed. 2016, 55, 1299.
doi: 10.1002/anie.201507796 pmid: 26662073 |
|
|
(d) Li, Z.; Hu, B.; Wu, Y.; Fei, C.; Deng, L. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 1730.
pmid: 26662073 |
|
|
(e) Trost, B. M.; Zell, D.; Hohn, C.; Mata, G.; Maruniak, A. Angew. Chem., Int. Ed. 2018, 57, 12916.
pmid: 26662073 |
|
|
(f) Trost, B. M.; Schultz, J. E.; Chang, T.; Maduabum, M. R. J. Am. Chem. Soc. 2019, 141, 9521.
pmid: 26662073 |
|
|
(g) Yang, S.-Q.; Wang, Y.-F.; Zhao, W.-C.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2021, 143, 7285.
pmid: 26662073 |
|
|
(h) Zhang, J.; Huo, X.; Xiao, J.; Zhao, L.; Ma, S.; Zhang, W. J. Am. Chem. Soc. 2021, 143, 12622.
pmid: 26662073 |
|
|
(i) Dai, J.; Li, L.; Ye, R.; Wang, S.; Wang, Y.; Peng, F.; Shao, Z. Angew. Chem., Int. Ed. 2023, 62, e202300756.
pmid: 26662073 |
|
| [38] |
(a) Miralles, N.; Maza, R. J.; Fernández, E. Adv. Synth. Catal. 2018, 360, 1306.
pmid: 33319887 |
|
(b) Nallagonda, R.; Padala, K.; Masarwa, A. Org. Biomol. Chem. 2018, 16, 1050.
doi: 10.1039/c7ob02978k pmid: 33319887 |
|
|
(c) Wu, C.; Wang, J. Tetrahedron Lett. 2018, 59, 2128.
pmid: 33319887 |
|
|
(d) Cuenca, A. B.; Fernández, E. Chem. Soc. Rev. 2021, 50, 72.
doi: 10.1039/d0cs00953a pmid: 33319887 |
|
|
(e) Corro, M.; Salvado, O.; González, S.; Dominguez-Molano, P.; Fernández, E. Eur. J. Inorg. Chem. 2021, 2802.
pmid: 33319887 |
|
|
(f) Jo, W.; Lee, J. H.; Cho, S. H. Chem. Commun. 2021, 57, 4346.
pmid: 33319887 |
|
|
(g) Lee, Y.; Han, S.; Cho, S. H. Acc. Chem. Res. 2021, 54, 3917.
pmid: 33319887 |
|
|
(h) Zhang, C.; Hu, W.; Morken, J. P. ACS Catal. 2021, 11, 10660.
pmid: 33319887 |
|
|
(i) Paul, S.; Das, K. K.; Aich, D.; Manna, S.; Panda, S. Org. Chem. Front. 2022, 9, 838.
pmid: 33319887 |
|
| [39] |
(a) Zhang, L.; Lovinǵer, G. J.; Edelstein, E. K.; Szymaniak, A. A.; Chierchia, M. P.; Morken, J. P. Science 2016, 351, 70.
pmid: 31062812 |
|
(b) Lovinger, G. J.; Aparece, M. D.; Morken, J. P. J. Am. Chem. Soc. 2017, 139, 3153.
doi: 10.1021/jacs.6b12663 pmid: 31062812 |
|
|
(c) Chierchia, M.; Law, C.; Morken, J. P. Angew. Chem., Int. Ed. 2017, 56, 11870.
doi: 10.1002/anie.201706719 pmid: 31062812 |
|
|
(d) Zhang, X.; Gao, C.; Morken, J. P. J. Am. Chem. Soc. 2023, 145, 16344.
pmid: 31062812 |
|
|
(e) For a review, see: Namirembe, S.; Morken, J. P. Chem. Soc. Rev. 2019, 48, 3464.
doi: 10.1039/c9cs00180h pmid: 31062812 |
|
| [40] |
(a) Davis, C. R.; Luvaga, I. K.; Ready, J. M. J. Am. Chem. Soc. 2021, 143, 4921.
|
|
(b) Davis, C. R.; Fu, Y.; Liu, P.; Ready, J. M. J. Am. Chem. Soc. 2022, 144, 16118.
|
|
| [41] |
(a) Ge, J.-F.; Zou, X.-Z.; Liu, X.-R.; Ji, C.-L.; Zhu, X.-Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2023, 62, e202307447.
|
|
(b) Chen, A.; Qiao, Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2023, 62, e202312605.
|
|
| [42] |
Jiang, X.-M.; Ji, C.-L.; Ge, J.-F.; Zhao, J.-H.; Zhu, X.-Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2024, 63, e202318441.
|
| [1] | 蒋旺, 史壮志. 芳烃间/对位选择性碳氢硼化反应研究进展[J]. 有机化学, 2023, 43(5): 1691-1705. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
