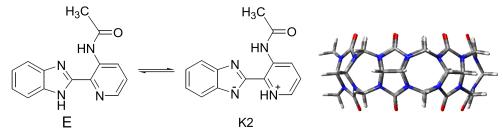
| [1] (a) Weller, A. Z. Elektrochemie 1956, 60, 1144. (b) Luque, A. M.; Mulder, W. H.; Calvente, J. J.; Cuesta, A.; Andreu, R. Anal. Chem. 2012, 84, 5778. (c) Presiado, I.; Gepshtein, R.; Erez, Y.; Huppert, D. J. Phys. Chem. A 2011, 115, 7591.[2] (a) Gould, E. A.; Popov, A. V.; Tolbert, L. M.; Presiado, I.; Erez, Y.; Huppert, D.; Solntsev, K. M. Phys. Chem. Chem. Phys. 2012. 14. 8964. (b) Thompson, W. H. J. Phys. Chem. B 2005, 109, 18201. (c) Gould, E. A.; Popov, A. V.; Tolbert, L. M.; Presiado, I.; Erez, Y.; Huppert, D.; Solntsev, K. M. Phys. Chem. Chem. Phys. 2012. 14. 8964. (d) Yi, P. G.; Liu, Z. J.; Wang, Z. X.; Hou, B.; Yu, X. Y.; Li, X. F. Acta Chim. Sinica 2012, 70, 27 (in Chinese). (易平贵, 刘峥军, 汪朝旭, 侯博, 于贤勇, 李筱芳, 化学学报, 2012, 70, 27.) (e) Yi, P. G.; Zhou, J. M.; Yu, X. Y.; Wang, Z. X.; Li, X. F.; Liu, Z. J.; Hou, B. Acta Chim. Sinica 2012, 70, 699 (in Chinese). (易平贵, 周继明, 于贤勇, 汪朝旭, 李筱芳, 刘峥军, 侯博, 化 学学报, 2012, 70, 699.) (f) Yi, P. G.; Zhou, J. M.; Wang, Z. X.; Yu, X. Y.; Li, X. F.; Chen, J.; Liu, Z. J. Acta Chim. Sinica 2012, 70, 1645 (in Chinese). (易平贵, 周继明, 汪朝旭, 于贤勇, 李筱芳, 陈建, 刘峥军, 化 学学报, 2012, 70, 1645.) (g) Yi, P. G.; Peng, H. L.; Wang, Z. X.; Yu, X. Y.; Li, X. F.; Liang, Y. H. Chin. J. Chem 2011, 29, 4, 650.[3] (a) Cui, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. J. Phys. Chem. C 2011, 115, 1824. (b) Parvari, G.; Reany, O.; Keinan, E. Isr. J. Chem. 2011, 51, 646. (c) Halterman, R.; Moore, J.; Yip, W. J. Fluoresc. 2011, 21, 1467.[4] (a) Fu, X. Z.; Huang, Y.; Tao, Z.; Xue, S. F.; Luo, C.; Ou, Y.; Zhang, J. X. Chin. J. Org. Chem. 2010, 30, 675 (in Chinese). (傅晓钟, 黄英, 陶朱, 薛赛凤, 罗春, 欧瑜, 张建新, 有机化学, 2010, 30, 675.) (b) Fan, Z.; Wang, J.; Huang, Y.; Xue, S.; Xiao, X.; Zhang, Y.; Zhu, Q.; Tao, Z. J. Inclusion Phenom. Macrocyclic Chem. 2012, 72, 213.[5] (a) Prabhu, A.; Siva, S.; Sankaranarayanan, R.; Rajendiran, N. J. Fluoresc. 2010, 20, 43. (b) Lee, Y.; Kwon, O.; Park, H. J.; Franz, J.; Jang, D. J. Photochem. Photobiol. A 2008, 194, 105. (c) Kim, Y.; Yoon, M.; Kim, D. J. Photochem. Photobiol. A 2001, 138, 167.[6] (a) Yi, P. G.; Wang, T.; Yu, X. Y.; Zhou, J. M.; Liu, R. H.; Wang, Z. X.; Zheng, B. S. Chin. J. Inorg. Chem. 2011, 27, 480 (in Chinese). (易平贵, 王涛, 于贤勇, 周继明, 刘容华, 汪朝旭, 郑柏树, 无 机化学学报, 2011, 27, 480.) (b) Yi, P. G.; Wang, T.; Zhou, J. M.; Peng, H. L.; Li, X. F.; Yu, X. Y.; Wang, Z. X. Acta Chim. Sinica 2009, 67, 2803 (in Chinese). (易平贵, 王涛, 周继明, 彭洪亮, 李筱芳, 于贤勇, 汪朝旭, 化 学学报, 2009, 67, 2803.) (c) Yi, P. G.; Yang, X. C.; Yu, X. Y; Liu, Z. J.; Liu, J.; Wang, Z. X. Chem. J. Chin. Univ. 2012, 33, 2657 (in Chinese). (易平贵, 阳习春, 于贤勇, 刘峥军, 刘金, 汪朝旭, 高等学校化 学学报, 2012, 33, 2657.)[7] Shaikh, M.; Dutta Choudhury, S.; Mohanty, J.; Bhasikuttan, A. C.; Nau, W. M.; Pal, H. Chem.-Eur. J. 2009, 15, 12362.[8] (a) Hammond, P. R. J. Chem. Soc., Pak. 1964, 479. (b) Yang, D. K.; Zeng, Z. J.; Chen, M. J.; Fan, S. W.; Yang, Y.; Li, M.; Lei, C. Y.; Jiang, L. S. Acta Chim. Sinica 2012, 70, 65 (in Chinese). (杨登科, 曾志坚, 陈木娟, 潘绍武, 杨宇, 李媚, 雷春燕, 蒋腊 生, 化学学报, 2012, 70, 65.)[9] (a) Stratmann, R. E.; Scuseria, G. E.; Frisch, M. J. J. Chem. Phys. 1998, 109, 8218. (b) Bauernschmitt, R.; Ahlrichs, R. Chem. Phys. Lett. 1996, 256, 454.[10] (a) Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Chem. Phys. Lett. 1998, 286, 253. (b) Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 106, 5151.[11] Benson, G. M.; Bleicher, K.; Chucholowski, A.; Dehmlow, H.; Grether, U.; Kuhn, B.; Martin, R. E.; Niesor, E. J.; Panday, N.; Richter, H.; Schuler, F.; Warot, X. M.; Wright, M.; Yang, M. US 7645785, 2010[Chem. Abstr. 2008, 148, 121703].[12] Ge, J.; Xue, S.; Zhu, Q.; Tao, Z.; Zhang, J. J. Inclusion Phenom. Macrocyclic 2007, 58, 63.[13] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, Jr., J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh PA, 2003. |