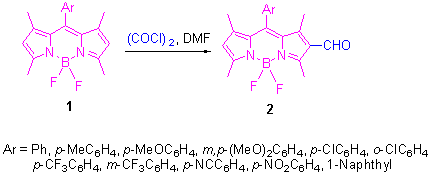
| [1] (a) Arbeloa, T. L.; Arbeloa, F. L.; Arbeloa, I. L.; Garcia-Moreno, I.; Costela, A.; Sastre, R.; Amat-Guerri, F. Chem. Phys. Lett. 1999, 299, 315.(b) Mula, S.; Ray, A. K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. J. Org. Chem. 2008, 73, 2146. [2] (a) Coskun, A.; Akkaya, E. U. J. Am. Chem. Soc. 2005, 127, 10464.(b) Yamada, K.; Nomura, Y.; Citterio, D.; Iwasawa, N.; Suzuki, K. J. Am. Chem. Soc. 2005, 127, 6956.(c) Wu, Y.; Peng, X.; Guo, B.; Fan, J.; Zhang, Z.; Wang, J.; Cui, A.; Gao, Y. Org. Biomol. Chem. 2005, 3, 1387.(d) Krumova, K.; Oleynik, P.; Karam, P.; Cosa, G. J. Org. Chem. 2009, 74, 3641.(e) Baruah, M.; Qin, W.; Basari?, N.; De Borggraeve, W. M.; Boens, N. J. Org. Chem. 2005, 70, 4152. [3] (a) Zeng, L.; Miller, E. W.; Pralle, A.; Isacoff, E. Y.; Chang, C. J. J. Am. Chem. Soc. 2006, 128, 10.(b) Wang, J.; Qian, X. Org. Lett. 2006, 8, 3721.(c) Yuan, M.; Zhou, W.; Liu, X.; Zhu, M.; Li, J.; Yin, X.; Zheng, H.; Zuo, Z.; Ouyang, C.; Liu, H.; Li, Y.; Zhu, D. J. Org. Chem. 2008, 73, 5008. [4] Ulrich, G.; Goeze, C.; Guardigli, M.; Roda, A.; Ziessel, R. Angew. Chem., Int. Ed. 2005, 44, 3694. [5] (a) Merino, E. J.; Weeks, K. M. J. Am. Chem. Soc. 2005, 127, 12766.(b) Li, Z.; Mintzer, E.; Bittman, R. J. Org. Chem. 2006, 71, 1718.(c) Meng, Q.; Kim, D. H.; Bai, X.; Bi, L.; Turro, N. J.; Ju, J. J. Org. Chem. 2006, 71, 3248.(d) Peters, C.; Billich, A.; Ghobrial, M.; Hoegenauer, K.; Ullrich, T.; Nussbaumer, P. J. Org. Chem. 2007, 72, 1842.(e) Li, Z.; Bittman, R. J. Org. Chem. 2007, 72, 8376. [6] (a) Atilgan, S.; Ekmekci, Z.; Dogan, A. L.; Guc, D.; Akkaya, E. U. Chem. Commun. 2006, 4398.(b) Lim, S. H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; van den Bergh, H.; Wagniéres, G.; Burgess, K.; Lee, H. B. J. Med. Chem. 2010, 53, 2865.(c) Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. J. Am. Chem. Soc. 2005, 127, 12162. [7] (a) Liu, J.-Y.; Yeung, H.-S.; Xu, W.; Li, X.; Ng, D. K. P. Org. Lett. 2008, 10, 5421.(b) Mula, S.; Elliott, K.; Harriman, A.; Ziessel, R. J. Phys. Chem. A 2010, 114, 10515. [8] (a) Hattori, S.; Ohkubo, K.; Urano, Y.; Sunahara, H.; Nagano, T.; Wada, Y.; Tkachenko, N. V.; Lemmetyinen, H.; Fukuzumi, S. J. Phys. Chem. B 2005, 109, 15368.(b) Erten-Ela, S.; Yilmaz, M. D.; Icli, B.; Dede, Y.; Icli, S.; Akkaya, E. U. Org. Lett. 2008, 10, 3299. [9] (a) Thivierge, C.; Bandichhor, R.; Burgess, K. Org. Lett. 2007, 9, 2135.(b) Shivran, N.; Mula, S.; Ghanty, T. K.; Chattopadhyay, S. Org. Lett. 2011, 13, 5870. (c) Ojida, A.; Sakamoto, T.; Inoue, M.-A.; Fujishima, S.-H.; Lippens, G.; Hamachi, I. J. Am. Chem. Soc. 2009, 131, 6543. [10] Jiao, L.; Yu, C.; Li, J.; Wang, Z.; Wu, M.; Hao, E. J. Org. Chem. 2009, 74, 7525. [11] Ye, J.-H.; Wang, G.; Huang, C.; Hu, Z.; Zhang, W.; Zhang, Y. Synthesis 2012, 104. [12] Alemdaroglu, F. E.; Alexander, S. C.; Ji, D.; Prusty, D. K.; B?rsch, M.; Herrmann, A. Macromolecules 2009, 42, 6529. [13] Gorman, A.; Killoran, J.; O'Shea, C.; Kenna, T.; Gallagher, W. M.; O'Shea, D. F. J. Am. Chem. Soc. 2004, 126, 10619. |