Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (9): 3617-3624.DOI: 10.6023/cjoc202103059 Previous Articles Next Articles
ARTICLES
王胜辉a, 关永风b, 刘秀娟c, 原信颖c, 蔚广曦a, 李银茹a, 张雁冰c, 宋健a,c,*( ), 李雯b,c,*(
), 李雯b,c,*( ), 张赛扬a,c,*(
), 张赛扬a,c,*( )
)
收稿日期:2021-03-30
修回日期:2021-05-25
发布日期:2021-06-29
通讯作者:
宋健, 李雯, 张赛扬
作者简介:基金资助:
Shenghui Wanga, Yongfeng Guanb, Xiujuan Liuc, Xinying Yuanc, Guangxi Yua, Yinru Lia, Yanbing Zhangc, Jian Songa,c( ), Wen Lib,c(
), Wen Lib,c( ), Saiyang Zhanga,c(
), Saiyang Zhanga,c( )
)
Received:2021-03-30
Revised:2021-05-25
Published:2021-06-29
Contact:
Jian Song, Wen Li, Saiyang Zhang
About author:Supported by:Share
Shenghui Wang, Yongfeng Guan, Xiujuan Liu, Xinying Yuan, Guangxi Yu, Yinru Li, Yanbing Zhang, Jian Song, Wen Li, Saiyang Zhang. Design, Synthesis and Anticancer Activity Studies of Novel Quinoline-Indole Derivatives[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3617-3624.
| Compound | IC50/(μmol•L–1)a | ||
|---|---|---|---|
| MGC-803 | Kyse450 | HCT-116 | |
| 9a | 3.91±0.22 | 3.27±0.18 | 3.11±0.16 |
| 9b | 0.58±0.01 | 0.68±0.03 | 0.59±0.04 |
| 9c | 4.10±0.21 | 5.17±0.53 | 8.23±0.42 |
| 9d | 2.86±0.19 | 6.78±1.04 | 6.01±0.37 |
| 9e | 4.34±0.44 | 4.68±0.24 | 5.91±0.38 |
| 9f | 7.00±0.82 | 5.27±0.34 | 11.1±1.41 |
| 9g | 2.42±0.12 | 3.04±0.23 | 2.87±0.21 |
| 9h | 1.16±0.05 | 1.40±0.12 | 1.71±0.19 |
| 9i | >20 | >20 | >20 |
| 9j | 4.58±0.25 | 6.57±0.21 | 5.06±0.33 |
| 5-Fu | 6.82±0.82 | 17.4±1.23 | 14.2±1.22 |
| Compound | IC50/(μmol•L–1)a | ||
|---|---|---|---|
| MGC-803 | Kyse450 | HCT-116 | |
| 9a | 3.91±0.22 | 3.27±0.18 | 3.11±0.16 |
| 9b | 0.58±0.01 | 0.68±0.03 | 0.59±0.04 |
| 9c | 4.10±0.21 | 5.17±0.53 | 8.23±0.42 |
| 9d | 2.86±0.19 | 6.78±1.04 | 6.01±0.37 |
| 9e | 4.34±0.44 | 4.68±0.24 | 5.91±0.38 |
| 9f | 7.00±0.82 | 5.27±0.34 | 11.1±1.41 |
| 9g | 2.42±0.12 | 3.04±0.23 | 2.87±0.21 |
| 9h | 1.16±0.05 | 1.40±0.12 | 1.71±0.19 |
| 9i | >20 | >20 | >20 |
| 9j | 4.58±0.25 | 6.57±0.21 | 5.06±0.33 |
| 5-Fu | 6.82±0.82 | 17.4±1.23 | 14.2±1.22 |
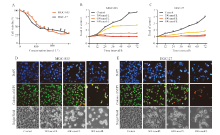

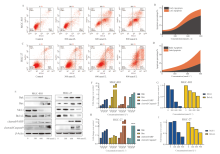
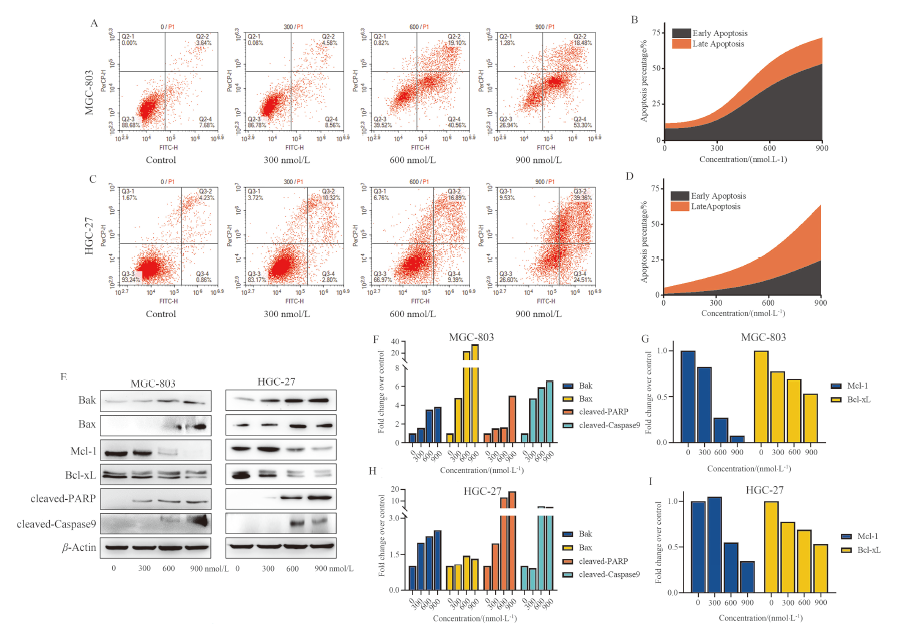

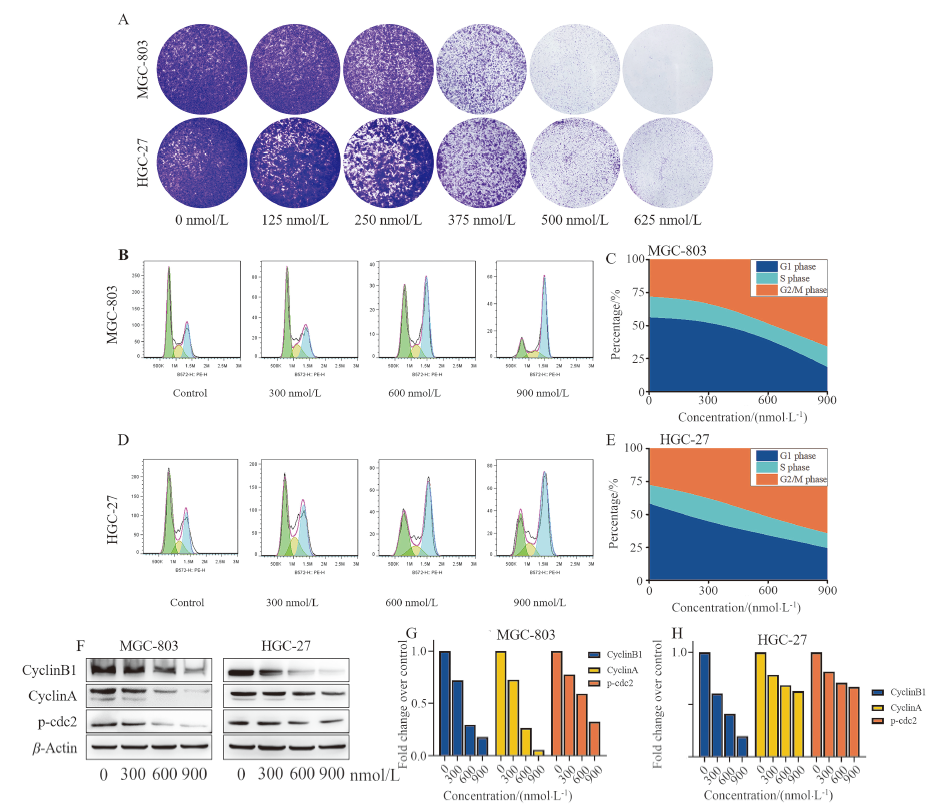
| [1] |
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Ca-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.v71.3 |
| [2] |
Boshuizen, J.; Peeper, D. S. Mol. Cell. 2020, 78, 1002.
doi: S1097-2765(20)30351-8 pmid: 32559422 |
| [3] |
Matada, B. S.; Pattanashettar, R.; Yernale, N. G. Bioorg. Med. Chem. 2021, 32, 115973.
doi: 10.1016/j.bmc.2020.115973 |
| [4] |
Yadav, P.; Shah, K. Bioorg. Chem. 2021, 109, 104639.
doi: 10.1016/j.bioorg.2021.104639 |
| [5] |
Afzal, O.; Kumar, S.; Haider, M. R.; Ali, M. R.; Kumar, R.; Jaggi, M.; Bawa, S. Eur. J. Med. Chem. 2015, 97, 871.
doi: 10.1016/j.ejmech.2014.07.044 |
| [6] |
Jain, S.; Chandra, V.; Kumar Jain, P.; Pathak, K.; Pathak, D.; Vaidya, A. Arabian J. Chem. 2019, 12, 4920.
doi: 10.1016/j.arabjc.2016.10.009 |
| [7] |
Jentsch, N. G.; Hart, A. P.; Hume, J. D.; Sun, J.; McNeely, K. A.; Lama, C.; Pigza, J. A.; Donahue, M. G.; Kessl, J. J. ACS Med. Chem. Lett. 2018, 9, 1007.
doi: 10.1021/acsmedchemlett.8b00269 |
| [8] |
Yang, S.-M.; Martinez, N. J.; Yasgar, A.; Danchik, C.; Johansson, C.; Wang, Y.; Baljinnyam, B.; Wang, A. Q.; Xu, X.; Shah, P.; Cheff, D.; Wang, X. S.; Roth, J.; Lal-Nag, M.; Dunford, J. E.; Oppermann, U.; Vasiliou, V.; Simeonov, A.; Jadhav, A.; Maloney, D. J. J. Med. Chem. 2018, 61, 4883.
doi: 10.1021/acs.jmedchem.8b00270 |
| [9] |
Zhong, C. C.; Chen, F.; Yang, J. L.; Jia, W. W.; Li, L.; Cheng, C.; Du, F. F.; Zhang, S. P.; Xie, C. Y.; Zhang, N. T.; Olaleye, O. E.; Wang, F. Q.; Xu, F.; Lou, L. G.; Chen, D. Y.; Niu, W.; Li, C. Acta Pharmacol. Sin. 2018, 39, 1048.
doi: 10.1038/aps.2017.199 pmid: 29620050 |
| [10] |
Golas, J. M.; Arndt, K.; Etienne, C.; Lucas, J.; Nardin, D.; Gibbons, J.; Frost, P.; Ye, F.; Boschelli, D. H.; Boschelli, F. Cancer Res. 2003, 63, 375.
|
| [11] |
Glen, H.; Mason, S.; Patel, H.; Macleod, K.; Brunton, V. G. BMC Cancer 2011, 11, 309.
doi: 10.1186/1471-2407-11-309 |
| [12] |
Gu, Y.-Y.; Lv, X.-Q.; Ma, X.-D.; Zhang, H.-J.; Ji, Y.-Y.; Ding, W.-Q.; Shen, L. Chin. J. Org. Chem. 2020, 40, 95. (in Chinese).
doi: 10.6023/cjoc201908021 |
|
( 顾依钰, 吕晓庆, 马晓东, 张浩健, 嵇媛媛, 丁婉婧, 沈立, 有机化学, 2020, 40, 95.)
|
|
| [13] |
Khelifi, I.; Naret, T.; Renko, D.; Hamze, A.; Alami, M. Eur. J. Med. Chem. 2017, 127, 1025.
doi: S0223-5234(16)30950-3 pmid: 28166995 |
| [14] |
Wu, B.-W.; Cui, X.-X.; Zhu, T.; Wang, S.-H.; Lu, C.-F.; Wang, J.-J.; Dang, H.-X.; Zhang, S.-Y.; Ding, L.-N.; Jin, C.-Y. Chin. J. Org. Chem. 2020, 40, 978. (in Chinese).
doi: 10.6023/cjoc201909016 |
|
( 吴博文, 崔鑫鑫, 朱挺, 王胜辉, 陆超凡, 王金杰, 党贺祥, 张赛扬, 丁丽娜, 金成允, 有机化学, 2020, 40, 978.)
|
|
| [15] |
Zhang, D.-Q.; Liu, X.; Pang, X.-J.; Liu, H.-M.; Zhang, Q.-R. Chin. J. Org. Chem. 2020, 41, 267. (in Chinese).
doi: 10.6023/cjo202006054 |
|
( 张丹青, 柳旭, 庞晓静, 刘宏民, 张秋荣, 有机化学, 2020, 41, 267.)
|
|
| [16] |
Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. Eur. J. Med. Chem. 2020, 203, 112506.
doi: S0223-5234(20)30478-5 pmid: 32688198 |
| [17] |
Thanikachalam, P. V.; Maurya, R. K.; Garg, V.; Monga, V. Eur. J. Med. Chem. 2019, 180, 562.
doi: 10.1016/j.ejmech.2019.07.019 |
| [18] |
Scuto, A.; Kirschbaum, M.; Kowolik, C.; Kretzner, L.; Juhasz, A.; Atadja, P.; Pullarkat, V.; Bhatia, R.; Forman, S.; Yen, Y.; Jove, R. Blood 2008, 111, 5093.
doi: 10.1182/blood-2007-10-117762 pmid: 18349321 |
| [19] |
Cross, D.; Ashton, S.; Nebhan, C.; Eberlein, C.; Finlay, M. R. V.; Hughes, G.; Jacobs, V.; Mellor, M.; Red Brewer, M.; Meador, C.; Orme, J.; Spitzler, P.; Powell, S.; Rahi, A.; Taylor, P.; Ward, R. A.; Daunt, P.; Galer, A.; Klinowska, T.; Richmond, G.; Pao, W. Mol. Cancer Ther. 2013, 12, A109.
|
| [20] |
Fu, D. J.; Cui, X. X.; Zhu, T.; Zhang, Y. B.; Hu, Y. Y.; Zhang, L. R.; Wang, S. H.; Zhang, S. Y. Bioorg. Chem. 2021, 107, 104634.
doi: 10.1016/j.bioorg.2021.104634 |
| [21] |
Li, W.; Shuai, W.; Sun, H.; Xu, F.; Bi, Y.; Xu, J.; Ma, C.; Yao, H.; Zhu, Z.; Xu, S. Eur. J. Med. Chem. 2019, 163, 428.
doi: 10.1016/j.ejmech.2018.11.070 |
| [22] |
Guo, T.; Liu, Y.; Zhao, Y. H.; Zhang, P. K.; Han, S. L.; Liu, H. M. Tetrahedron Lett. 2016, 57, 4629
|
| [23] |
Hu, Y.; Li, Z.-Y.; Ding, Y.-J.; Li, Z.-Y.; Liu, Z.-Y.; Shen, Y.-M. Chin. J. Org. Chem. 2019, 39, 3230. (in Chinese).
doi: 10.6023/cjoc201905013 |
|
( 胡园, 李震宇, 丁艳娇, 李志颖, 刘志勇, 沈月毛, 有机化学, 2019, 39, 3230.)
|
|
| [24] |
Yuan, S.; Yu, B.; Liu, H. M. Adv. Synth. Catal. 2018, 361, 59.
doi: 10.1002/adsc.v361.1 |
| [25] |
Jian, S.; Gao, Q.-L.; Wu, B.-W.; Li, D.; Shi, L.; Zhu, T.; Lou, J.-F.; Jin, C.-Y.; Zhang, Y.-B.; Zhang, S.-Y.; Liu, H.-M. Eur. J. Med. Chem. 2019, 183, 111731.
doi: S0223-5234(19)30883-9 pmid: 31577977 |
| [1] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [2] | Wenfeng Bei, Jian Pan, Dongmei Ran, Yilin Liu, Zhen Yang, Ruokun Feng. Cobalt-Catalyzed [4+2] Annulation of Indole Carboxamide with Diynes and Monoacetylene: Direct Access to γ-Carbolinones [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3226-3238. |
| [3] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [4] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [5] | Yingke Feng, He Wang, Mengxing Cui, Ran Sun, Xin Wang, Yang Chen, Lei Li. Visible-Light-Induced Difluoroalkylated Cyclization of Novel Functionalized Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2913-2925. |
| [6] | Yi Wang, Jian Zhang, Yangzi Liu, Xiaoyan Luo, Weiping Deng. Palladium-Catalyzed Asymmetric [3+4] Cycloadditions for the Construction of Cyclohepta[b]indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2864-2877. |
| [7] | Weishu Zhang, Lifei Nie, Khurshed Bozorov, Haji Akber Aisa, Jiangyu Zhao. Synthesis of Diethyl 2,5-Diaminothiophene-3,4-dicarboxylate Derivatives and Antitumor Activity Study [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2543-2552. |
| [8] | Yue Zhu, Lu Chen, Jing Zhao, Qingrong Sun, Weiqing Yang, Haiyan Fu, Menglin Ma. Synthesis of Quinoline Derivatives by Friedländer Reaction Catalyzed by Ruthenium Complexes of Substituted 8-Hydroxyquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2528-2542. |
| [9] | Lixing Sun, Tingting Sun, Haiqing Wang, Shufang Wu, Xiaoye Wang, Tianya Liu, Yuchen Zhang. Lewis Acid-Catalyzed [2+4] Cyclization of 3-Alkyl-2-vinylindoles with α,β-Unsaturated N-Sulfonyl Ketimines [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2178-2188. |
| [10] | Zeren Sun, Bingxin Zhai, Guangchao He, Hui Shen, Linya Chen, Shan Zhang, Yi Zou, Qihua Zhu, Yungen Xu. Synthesis and Anti-inflammatory Evaluation of Novel 1,2,3-Triazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2143-2155. |
| [11] | Weina Tian, Liang Xu, Yu Wei, Pengfei Li. Synthesis of Isoquinoline-3-carboxylate Chelated B,B-Diaryl Tetracoordinated Organoboron Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1792-1798. |
| [12] | Jun Lu, Qichuang Li, Renxiao Liang, Yixia Jia. Nickel-Catalyzed Intramolecular Dearomative Arylation of Pyridiniums and Quinoliniums [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1875-1882. |
| [13] | Mingyang Pang, Honghong Chang, Zhang Feng, Juan Zhang. Recent Advances in Transition-Metal-Catalyzed Tandem Dearomatization of Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1271-1291. |
| [14] | Hua Huang, Xin Li, Jianke Su, Qiuling Song. Difluorocarbene-Enabled Synthesis of 3-Substituted-2-oxoindoles from o-Vinylanilines [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1146-1156. |
| [15] | Jinxiao Zhao, Tonghui Wei, Sen Ke, Yi Li. Visible Light-Catalyzed Synthesis of Difluoroalkylated Polycyclic Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1102-1114. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||