Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (11): 3258-3263.DOI: 10.6023/cjoc201902031 Previous Articles Next Articles
收稿日期:2019-02-26
发布日期:2019-07-03
通讯作者:
李江
E-mail:lijiang@cup.edu.cn
基金资助:
Li Jianga*( ), Wan Tonga, Zhang Junjiea, Fu Yaob
), Wan Tonga, Zhang Junjiea, Fu Yaob
Received:2019-02-26
Published:2019-07-03
Contact:
Li Jiang
E-mail:lijiang@cup.edu.cn
Supported by:Share
Li Jiang, Wan Tong, Zhang Junjie, Fu Yao. Iron-Catalyzed Selective Hydrogenation of Stearic Acid to Stearyl Alcohol[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3258-3263.
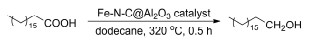 | |||||||
| Entry | Catalyst | Conv./% | Yield b/% | Carbon balance | |||
| C18-OH | C17 | C18 | C17-CHO | ||||
| 1 | — | 33.3 | 0.5 | 0.5 | 0.1 | 0.2 | 3.9 |
| 2 | 20% Fe-N-C@Al2O3-900 | >99 | 88.6 | 1.1 | 2.4 | 0.4 | 92.5 |
| 3 | 20% Fe-Al2O3-900 | 21.6 | 0.9 | 0.9 | 0.1 | 2.2 | 19.0 |
| 4 | N-C@Al2O3-900 | 42.3 | 0.5 | 0.5 | 0.4 | 0.4 | 4.3 |
| 5 | Al2O3 | 43.3 | 7.9 | 1.0 | 0.9 | 4.1 | 32.1 |
| 6 | 20% Fe-N-C@Al2O3-550 | 98.3 | 80.4 | 1.5 | 14.3 | 0 | 97.9 |
| 7 | 20% Fe-N-C@Al2O3-700 | 95.7 | 34.1 | 0.7 | 3.8 | 3.0 | 43.5 |
| 8 | 20% Fe-N-C@Al2O3-1100 | 97.1 | 47.9 | 1.6 | 11.8 | 2.6 | 65.8 |
| 9 | 20% Fe-N-C@SiO2-900 | 88.5 | 1.0 | 1.3 | 0.1 | 1.1 | 4.0 |
| 10 | 20% Fe-N-C-900 | 92.1 | 4.3 | 1.6 | 0.7 | 1.8 | 9.1 |
| 11 | 20% Fe-N-C@TiO2-900 | 66.9 | 3.6 | 0.9 | 0.4 | 3.0 | 11.8 |
| 12 | 20% Fe(NO3)3-N-C@Al2O3-900 | 80.6 | 10.8 | 1.2 | 0.6 | 2.2 | 18.4 |
| 13 | 20% Fe-phen-C@Al2O3-900 | 96.2 | 58.8 | 0.9 | 4.4 | 3.5 | 70.3 |
| 14 | 3% Fe-N-C@Al2O3-900 | 89.4 | 52.6 | 1.0 | 1.1 | 2.9 | 64.4 |
| 15 | 5% Fe-N-C@Al2O3-900 | 94.7 | 21.6 | 0.6 | 0.8 | 3.9 | 28.4 |
| 16 | 10% Fe-N-C@Al2O3-900 | 98.0 | 62.0 | 0.6 | 0.6 | 1.8 | 66.3 |
| 17 | 30% Fe-N-C@Al2O3-900 | 99.3 | 46.0 | 0.8 | 1.5 | 2.2 | 50.9 |
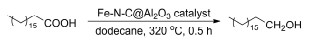 | |||||||
| Entry | Catalyst | Conv./% | Yield b/% | Carbon balance | |||
| C18-OH | C17 | C18 | C17-CHO | ||||
| 1 | — | 33.3 | 0.5 | 0.5 | 0.1 | 0.2 | 3.9 |
| 2 | 20% Fe-N-C@Al2O3-900 | >99 | 88.6 | 1.1 | 2.4 | 0.4 | 92.5 |
| 3 | 20% Fe-Al2O3-900 | 21.6 | 0.9 | 0.9 | 0.1 | 2.2 | 19.0 |
| 4 | N-C@Al2O3-900 | 42.3 | 0.5 | 0.5 | 0.4 | 0.4 | 4.3 |
| 5 | Al2O3 | 43.3 | 7.9 | 1.0 | 0.9 | 4.1 | 32.1 |
| 6 | 20% Fe-N-C@Al2O3-550 | 98.3 | 80.4 | 1.5 | 14.3 | 0 | 97.9 |
| 7 | 20% Fe-N-C@Al2O3-700 | 95.7 | 34.1 | 0.7 | 3.8 | 3.0 | 43.5 |
| 8 | 20% Fe-N-C@Al2O3-1100 | 97.1 | 47.9 | 1.6 | 11.8 | 2.6 | 65.8 |
| 9 | 20% Fe-N-C@SiO2-900 | 88.5 | 1.0 | 1.3 | 0.1 | 1.1 | 4.0 |
| 10 | 20% Fe-N-C-900 | 92.1 | 4.3 | 1.6 | 0.7 | 1.8 | 9.1 |
| 11 | 20% Fe-N-C@TiO2-900 | 66.9 | 3.6 | 0.9 | 0.4 | 3.0 | 11.8 |
| 12 | 20% Fe(NO3)3-N-C@Al2O3-900 | 80.6 | 10.8 | 1.2 | 0.6 | 2.2 | 18.4 |
| 13 | 20% Fe-phen-C@Al2O3-900 | 96.2 | 58.8 | 0.9 | 4.4 | 3.5 | 70.3 |
| 14 | 3% Fe-N-C@Al2O3-900 | 89.4 | 52.6 | 1.0 | 1.1 | 2.9 | 64.4 |
| 15 | 5% Fe-N-C@Al2O3-900 | 94.7 | 21.6 | 0.6 | 0.8 | 3.9 | 28.4 |
| 16 | 10% Fe-N-C@Al2O3-900 | 98.0 | 62.0 | 0.6 | 0.6 | 1.8 | 66.3 |
| 17 | 30% Fe-N-C@Al2O3-900 | 99.3 | 46.0 | 0.8 | 1.5 | 2.2 | 50.9 |


| [1] |
Huber G. W. Iborra S. Corma A. Chem. Rev. 2006 106 4044.
doi: 10.1021/cr068360d |
| [2] |
Corma A. Iborra S. Velty A. Chem. Rev. 2007 107 2411.
doi: 10.1021/cr050989d |
| [3] |
Yang Z. Fu Y. Guo Q. Chin. J. Org. Chem. 2015 35 273.
doi: 10.6023/cjoc201409012 |
|
杨 珍 傅 尧 郭 庆祥 有机化学 2015 35 273.
doi: 10.6023/cjoc201409012 |
|
| [4] |
Jamil F. Al-Haj L. Al-Muhtaseb A. H. Al-Hinai M. A. Baawain M. Rashid U. Ahmad M. N. M. Rev. Chem. Eng. 2018 34 267.
doi: 10.1515/revce-2016-0026 |
| [5] | Besson, M.; Gallezot, P.; Pinel, C. Chem. Rev. 2014, 114, 1827. |
| [6] | Gupta S. Frost and Sullivan Market Insight 2004 11075449. |
| [7] |
Adkins H. Folkers K. J. Am. Chem. Soc. 1931 53 1095.
doi: 10.1021/ja01354a042 |
| [8] |
Toba M. Tanaka S.-I. Niwa S.-I. Mizukami F. Koppány Z. Guczi L. Cheah K.-Y. Tang T.-S. Appl. Catal., A 1999 189 243.
doi: 10.1016/S0926-860X(99)00281-1 |
| [9] |
Mendes M. Santos O. Jordao E. Silva A. Appl. Catal., A 2001 217 253.
doi: 10.1016/S0926-860X(01)00613-5 |
| [10] |
Takeda Y. Nakagawa Y. Tomishige K. Catal. Sci. Technol. 2012 2 2221.
doi: 10.1039/c2cy20302b |
| [11] |
Takeda Y. Tamura M. Nakagawa Y. Okumura K. Tomishige K. ACS Catal. 2015 5 7034.
doi: 10.1021/acscatal.5b01054 |
| [12] |
Ullrich J. Breit B. ACS Catal. 2018 8 785.
doi: 10.1021/acscatal.7b03484 |
| [13] |
Manyar H. G. Paun C. Pilus R. Rooney D. W. Thompson J. M. Hardacre C. Chem. Commun. 2010 46 6279.
doi: 10.1039/c0cc01365j |
| [14] |
Rozmysłowicz B. Kirilin A. Aho A. Manyar H. Hardacre C. Wärn J. Salmi T. Murzin D. Y. J. Catalysis 2015 328 197.
doi: 10.1016/j.jcat.2015.01.003 |
| [15] |
Toyao T. Siddiki S. M. Touchy A. S. Onodera W. Kon K. Morita Y. Kamachi T. Yoshizawa K. Shimizu K. I. Chem.-Eur. J. 2017 23 1001.
doi: 10.1002/chem.201604762 |
| [16] |
Kandel K. Chaudhary U. Nelson N. C. Slowing I. I. ACS Catal. 2015 5 6719.
doi: 10.1021/acscatal.5b01664 |
| [17] |
Wu L. Li L. Li B. Zhao C. Chem. Commun. 2017 53 6152.
doi: 10.1039/C7CC01126A |
| [18] |
Onyestyák G. Harnos S. Kalló D. Catal. Commun. 2011 16 184.
doi: 10.1016/j.catcom.2011.09.032 |
| [19] |
Gao X. Tong D. Zhong H. Jin B. Jin F. Zhang H. RSC Adv. 2016 6 27623.
doi: 10.1039/C6RA01150K |
| [20] |
Kong X. Fang Z. Bao X. Wang Z. Mao S. Wang Y. J. Catalysis 2018 367 139.
doi: 10.1016/j.jcat.2018.08.022 |
| [21] |
Jia W. Xu G. Liu X. Zhou F. Ma H. Zhang Y. Fu Y. Energy Fuels 2018 32 8438.
doi: 10.1021/acs.energyfuels.8b01114 |
| [22] |
Song S. Wang D. Di L. Wang C. Dai W. Wu G. Guan N. Li L. Chin. J. Catal. 2018 39 250.
doi: 10.1016/S1872-2067(17)63003-1 |
| [23] |
Li J. Zhang J. Wang S. Xu G. Wang H. Vlachos D. G. ACS Catal. 2019 9 1564.
doi: 10.1021/acscatal.8b04967 |
| [24] |
Jagadeesh R. V. Surkus A. Junge H. Pohl M. Radnik J. Rabeah J. Huan H. Schunemann V. Bruckner A. Beller M. Science 2013 342 1073.
doi: 10.1126/science.1242005 |
| [25] |
Cui X. Li Y. Bachmann S. Scalone M. Surkus A. Junge K. Topf C. Beller M. J. Am. Chem. Soc. 2015 137 10652.
doi: 10.1021/jacs.5b05674 |
| [26] |
Natte K. Neumann H. Jagadeesh R. V. Beller M. Nat. Commun. 2017 8 1344.
doi: 10.1038/s41467-017-01428-0 |
| [27] |
Li J. Liu J. Zhou H. Fu Y. ChemSusChem 2016 9 1339.
doi: 10.1002/cssc.201600089 |
| [28] |
Li J. Liu J. Liu H. Xu G. Zhang J. Liu J. Zhou G. Li Q. Xu Z. Fu Y. ChemSusChem 2017 10 1436.
doi: 10.1002/cssc.201700105 |
| [29] |
Li J. Zhang J. Liu H. Liu J. Xu G. Liu J. Sun H. Fu Y. ChemistrySelect 2017 2 11062.
doi: 10.1002/slct.201701966 |
| [30] |
Li J. Sun H. Liu J. X. Zhang J. J. Li Z. X. Fu Y. Mol. Catal. 2018 452 36.
doi: 10.1016/j.mcat.2018.03.014 |
| [31] |
Leveneur J. Waterhouse G. I. N. Kennedy J. Metson J. B. Mitchell D. R. G. J. Phys. Chem. C 2011 115 20978.
doi: 10.1021/jp206357c |
| [1] | Wei Zhang, Huan Xia, Xin Cao, Binyu Xu, Zhengyun Li. Research Progress on Hydrogel Electrolytes for Flexible Zinc-Ion Batteries [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 148-158. |
| [2] | Zhangtao Zhou, Yang Wang, Bingxin Cheng, Weiping Ye. [RuCl(p-cymene)-(S)-BINAP]Cl Catalyzed Asymmetric Preparation of trans-3-Amino-bicyclo[2.2.2]octane-2-carboxylic Acid Ethyl Ester [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2961-2967. |
| [3] | Xiangping Chen, Chenxiang Meng, Mengna Li, Shangmin Chu, Xinxin Zhu, Kai Xu, Lantao Liu, Tao Wang, Fenghua Zhang, Fei Li. Fe-Catalyzed Synthesis of Sulfide-Based Aromatic Primary Amines in Water Promoted by Sodium-Ascorbate [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2800-2807. |
| [4] | Yangyang Chu, Zhaobin Han, Kuiling Ding. Progresses in the Application of Kinetic Resolution in Transition Metal Catalyzed Asymmetric (Transfer) Hydrogenation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1934-1951. |
| [5] | Shengjie Jiang, Yang Wang, Xin Xu. Rare-Earth Metal Complexes-Catalyzed Dehydropolymerization of Methylamine-Boranes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1786-1791. |
| [6] | Shuang Liu, Lianghua Zou, Xiaoming Wang. Advance of Dehydrogenation and Transfer Hydrogenation of Ammonia-Borane Catalyzed by Homogeneous Cobalt Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1713-1725. |
| [7] | Qian Dou, Taimin Wang, Lijing Fang, Hongbin Zhai, Bin Cheng. Recent Development of Photoinduced Iron-Catalysis in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1386-1415. |
| [8] | Yanjie Qu, Yajun Li, Hongli Bao. Research Progress of Reaction-Based Probes for Detecting Fluoride Ion [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 809-825. |
| [9] | Wei Sun, Shoufei Zhu. Hydrosilylation Reactions of Alkene with Tertiary Silanes Catalyzed by Iron-Series Metals [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3339-3351. |
| [10] | Shouyi Cen, Zhipeng Zhang. Synthesis of Biphenanthrol-Based Confined Chiral Phosphoric Acid [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2574-2581. |
| [11] | Tingshu Cao, Xiangyang Wei, Min Luo, Yifei Wang, Zijun Pan, Cheng Xu, Guodong Yin. PhI(OAc)2-Promoted Dehydrogenation Oxidation for the Synthesis of 2-(Aryl/alkylthio)phenols and 10H-Phenothiazines [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2079-2088. |
| [12] | Tongli Zhang, Jun Yan, Jingli He, Xuezhen Kou, Jiefeng Shen, Delong Liu, Wanbin Zhang. Synthesis of Chiral 5-Aryl-2-oxazolidinones via an Ir-BiphPHOX Catalyzed Enantioselective Hydrogenation [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1747-1758. |
| [13] | Wenjian Zhou, Xinrui Xiao, Yonghong Liu, Xu Zhang. Magnetic Se/Fe/PCN-Catalyzed Oxidative Cracking Alkenes in O2 [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1849-1855. |
| [14] | Xinyao Wang, Qingqing Zhang, Shuyang Liu, Min Li, Haifang Li, Chunying Duan, Yunhe Jin. Visible Light-Induced Metal-Free Benzylation of Quinones via Cross Dehydrogenation Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1443-1452. |
| [15] | Miaomiao Zhang, Bo Han, Haojie Ma, Liang Zhao, Jijiang Wang, Yuqi Zhang. Hydrosilanes as Hydrogen Source: Iridium-Catalyzed Hydrogenation of N-Heteroarenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1170-1178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||