化学学报 ›› 2012, Vol. 70 ›› Issue (17): 1839-1846.DOI: 10.6023/A12050171 上一篇 下一篇
研究论文
张蕴a,b, 奚晓青a, 许姗妮a, 周俊晨a, 周津金a, 徐启宏a, 沈昊宇a
Zhang Yuna,b, Xi Xiaoqinga, Xu Shannia, Zhou Junchena, Zhou Jinjina, Xu Qihonga, Shen Haoyua
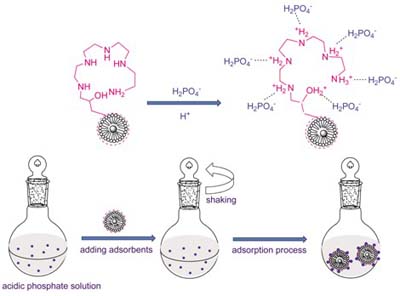
合成了4种氨基(乙二胺(EDA), 二乙烯三胺(DETA), 三乙烯四胺(TETA)和四乙烯五胺(TEPA))功能化纳米复合材料(NH2-NCMs, 分别命名为EDA-NCMs, DETA-NCMs, TETA-NCMs和TEPA-NCMs). 采用FTIR(傅立叶变换红外光谱分析), TG/DTG(热重差热分析), XPS(X-射线光电子能谱分析)等手段对其进行了表征, 并考察了其对水中磷酸盐的吸附性能. 结果表明: 溶液pH对其吸附性能影响较大, 在pH为2.5的条件下, 4种吸附剂对磷酸盐的吸附效果最佳; 在5 min内即可达到平衡吸附量的90%; 4种吸附剂对磷酸盐的吸附均符合Langmuir等温吸附模型; 吸附行为均符合准二级速率模型; 吸附反应为自发进行的放热反应和熵减的过程; 推测其吸附机理以静电作用力为主. 4种吸附剂用于磷酸盐含量为50 mg/L的废水处理的吸附率均大于97%, 均达到一级A出水的排放标准(≤1.53 mg/L, 以PO43-计).