| [1] Rauhut, M. M.; Currier, H. US 3074999, 1963[Chem. Abstr. 1963, 58, 11224a].
[2] For selected reviews on the R-C reaction see: (a) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035; (b) Aroyan, C. E.; Dermenci, A.; Miller, S. J. Tetrahedron 2009, 65, 4069; (c) Xie, P.; Huang, Y. Eur. J. Org. Chem. 2013, 6213; (d) Bharadwaj, K. C. RSC Adv. 2015, 5, 75923.
[3] For references on the application of the R-C reaction in total synthesis see: (a) Ergüden, J.-K.; Moore, H. W. Org. Lett. 1999, 1, 375; (b) Mergott, D. J.; Frank, S. A.; Roush, W. R. Org. Lett. 2002, 4, 3157; (c) Agapiou, K.; Krische, M. J. Org. Lett. 2003, 5, 1737; (d) Mergott, D. J.; Frank, S. A.; Roush, W. R. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 11955; (e) Stark, L. M.; Pekari, K.; Sorensen, E. J. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12064; (f) Winbush, S. M.; Mergott, D. J.; Roush, W. R. J. Org. Chem. 2008, 73, 1818; (g) Dermenci, A.; Selig, P. S.; Domaoal, R. A.; Spasov, K. A.; Anderson, K. S.; Miller, S. J. Chem. Sci. 2011, 2, 1568.
[4] For selected progress in the enantioselective intramolecular R-C reaction, see: (a) Aroyan, C. E.; Miller, S. J. J. Am. Chem. Soc. 2007, 129, 256; (b) Aroyan, C. E.; Dermenci, A.; Miller, S. J. J. Org. Chem. 2010, 75, 5784; (c) Osuna, S.; Dermenci, A.; Miller, S. J.; Houk, K. N. Chem. Eur. J. 2013, 19, 14245; (d) Marqués-López, E.; Herrera, R. P.; Marks, T.; Jacobs, W. C.; Könning, D.; de Figueiredo, R. M.; Christmann, M. Org. Lett. 2009, 11, 4116; (e) Wang, X.-F.; Peng, L.; An, J.; Li, C.; Yang, Q.-Q.; Lu, L.-Q.; Gu, F.-L.; Xiao, W.-J. Chem. Eur. J. 2011, 17, 6484; (f) Gong, J.-J.; Li, T.-Z.; Pan, K.; Wu, X.-Y. Chem. Commun. 2011, 47, 1491; (g) Zhang, X.-N.; Shi, M. Eur. J. Org. Chem. 2012, 6271; (h) Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Enders, D.; Sasai, H. Angew. Chem., Int. Ed. 2012, 51, 5423; (i) Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Suzuki, M.; Enders, D.; Sasai, H. Tetrahedron 2013, 69, 1202; (j) Jin, Z.; Yang, R.; Du, Y.; Tiwari, B.; Ganguly, R.; Chi, Y. R. Org. Lett. 2012, 14, 3226; (k) Scanes, R. J. H.; Grossmann, O.; Grossmann, A.; Spring, D. R. Org. Lett. 2015, 17, 2462.
[5] For selected reports on cross RC reactions see: (a) Jih, R. H.; Hakimelahi, G. H.; Chou, C.-T. Tetrahedron Lett. 1992, 33, 6469; (b) Reynolds, T. E.; Binkley, M. S.; Scheidt, K. A. Org. Lett. 2008, 10, 2449; (c) Kumar, R.; Kumar, T.; Mobin, S. M.; Nambothiri, I. N. N. J. Org. Chem. 2013, 78, 5073; (d) Shanbhag, P.; Nareddy, P. R.; Dadwal, M.; Mobin, S. M.; Namboothiri, I. N. N. Org. Biomol. Chem. 2010, 8, 4867; (e) Zhou, R.; Wang, J.; Yu, J.; He, Z. J. Org. Chem. 2013, 78, 10596.
[6] For selected reports on domino cyclization initiated by cross R-C reactions see: (a) Sun, X.; Sengupta, S.; Peterson, J. L.; Wang, H.; Lewis, J. P.; Shi, X. Org. Lett. 2007, 9, 4495; (b) Zhong, C.; Chen, Y.; Petersen, J. L.; Akhmedov, N. G.; Shi, X. Angew. Chem., Int. Ed. 2009, 48, 1279; (c) Yao, W.; Wu, Y.; Wang, G.; Zhang, Y.; Ma, C. Angew. Chem., Int. Ed. 2009, 48, 9713; (d) Ma, J.; Xie, P.; Hu, C.; Huang, Y.; Chen, R. Chem. Eur. J. 2011, 17, 7418; (e) Liu, W.; Zhou, J.; Zheng, C.; Chen, X.; Xiao, H.; Yang, Y.; Guo, Y.; Zhao, G. Tetrahedron 2011, 67, 1768; (f) Xie, P.; Huang, Y.; Lai, W.; Meng, X.; Chen, R. Org. Biomol. Chem. 2011, 9, 6707; (g) Shi, Z.; Tong, Q.; Leong, W. W. Y.; Zhong, G. Chem. Eur. J. 2012, 18, 9802; (h) Shi, Z.; Yu, P.; Loh, T.-P.; Zhong, G. Angew. Chem. Int. Ed. 2012, 51, 7825; (i) Hu, C.; Geng, Z.; Ma, J.; Huang, Y.; Chen, R. Chem. Asian J. 2012, 7, 2032; (j) Hu, C.; Zhang, Q.; Huang, Y. Chem. Asian J. 2013, 8, 1981; (k) Shi, Z.; Loh, T.-P. Angew. Chem., Int. Ed. 2013, 52, 8554; (l) Peng, J.; Huang, X.; Zheng, P.-F.; Chen, Y.-C. Org. Lett. 2013, 15, 5534; (m) Zhang, Y.-Y.; Gurubrahamam, R.; Chen, K. Adv. Synth. Catal. 2015, 357, 2457.
[7] Zhao, Q.-Y.; Pei, C.-K.; Guan, X.-Y.; Shi, M. Adv. Synth. Catal. 2011, 353, 1973.
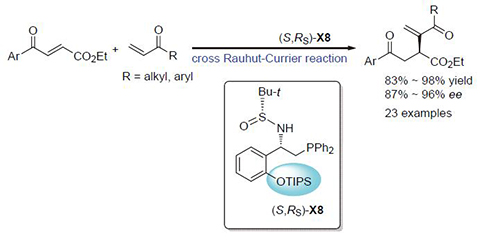
[8] Dong, X.; Liang, L.; Li, E.; Huang, Y. Angew. Chem., Int. Ed. 2015, 54, 1621.
[9] (a) Zhang, Z.-M.; Chen, P.; Li, W.; Niu, Y.; Zhao, X.-L.; Zhang, J. Angew. Chem., Int. Ed. 2014, 53, 4350; (b) Su, X.; Zhou, W.; Li, Y.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 6874; (c) Zhou, W.; Su. X.; Tao, M.; Zhu, C.; Zhao, Q.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 14853; (d) Chen, P.; Su, X.; W.; Xiao, Y.; Zhang, J. Tetrahedron 2016, 72, 2700; (e) Zhou, W.; Chen, P.; Tao, M.; Su, X.; Zhao, Q.; Zhang, J. Chem. Commun. 2016, 52, 7612; (f) Zhang, Z.-M.; Xu, B.; Xu, S.; Wu, H.-H.; Zhang, J. Angew. Chem., Int. Ed. 2016, 55, 6324; (g) Hu, H.; Wang, Y.; Qian, D.; Zhang, Z.-M.; Liu, L.; Zhang, J. Org. Chem. Front. 2016, 3, 759.
[10] For reviews related to chiral phosphines catalysis, see: (a) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140; (b) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005; (c) Marinetti, A.; Voituriez, A. Synlett 2010, 174; (d) Wang, S.-X.; Han, X.; Zhong, F.; Wang, Y.; Lu, Y. Synlett 2011, 2766; (e) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724; (f) Xu, L.-W. ChemCatChem 2013, 5, 2775; (g) Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659; (h) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588; (i) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927; (j) Wei, Y.; Shi, M. Chem. Asian J. 2014, 9, 2720; (k) Li, W.; Zhang, J. Chem. Soc. Rev. 2016, 45, 1657; (l) Yang, L.; Ma, J. Acta Chim. Sinica 2016, 74, 130(杨丽军, 马军安, 化学学报, 2016, 74, 130.); (m) Zhao, W.-X.; Yang, D.-Y.; Zhang, Y.-H. Chin. J. Org. Chem. 2016, 36, DOI: 10. 6023/ cjoc201603006. (赵文献, 杨代月, 张玉华, 有机化学, 2016, 36, DOI:10.6023/cjoc201603006.)
[11] For selected asymmetric β-aminephosphine catalysis, see: (a) Fang, Y.-Q.; Jacobsen, E. N. J. Am. Chem. Soc. 2008, 130, 5660; (b) Xiao, H.; Chai, Z.; Zheng, C.-W.; Yang, Y.-Q.; Liu, W.; Zhang, J.-K.; Zhao, G. Angew. Chem., Int. Ed. 2010, 49, 4467; (c) Han, X.; Wang, Y.; Zhong, F.; Lu, Y. J. Am. Chem. Soc. 2011, 133, 1726; (d) Zhong, F.; Luo, J.; Chen, G.-Y.; Dou, X.; Lu, Y. J. Am. Chem. Soc. 2012, 134, 10222; (e) Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Chem. Sci. 2012, 3, 1231; (f) Zhong, F.; Dou, X.; Han, X.; Yao, W.; Zhu, Q.; Meng, Y.; Lu, Y. Angew. Chem., Int. Ed. 2013, 52, 943; (g) Fang, Q.; Tadross, P. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2014, 136, 17966; (h) Han, X.; Yao, W.; Wang, T.; Tan, Y. R.; Yan, Z.; Kwiatkowski, J.; Lu, Y. Angew. Chem., Int. Ed. 2014, 53, 5643. (i) Fang, Y.-Q.; Tadross, P. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2014, 136, 17966; (j) Henry, C. E.; Xu, Q.; Fan, Y. C.; Martin, T. J.; Belding, L.; Dudding, T.; Kwon, O. J. Am. Chem. Soc. 2014, 136, 11890; (k) Yao, W.; Dou, X.; Lu, Y. J. Am. Chem. Soc. 2015, 137, 54; (l) Wang, H.-Y.; Zhang, K.; Zhang, C.-W.; Chai, Z.; Cao, D.-D.; Zhang, J.-X.; Zhao, G. Angew. Chem., Int. Ed. 2015, 54, 1775; (m) Li, Y.; Xiao, S.; Zhou, W.; Li, W.; Zhang, J. Chem. Eur. J. 2015, 21, 4224; (n) Lou, Y.-P.; Zheng, C.-W.; Pang, R.-M.; Jin, Q.-W.; Zhao, G.; Li, Z. Org. Lett. 2015, 17, 688; (o) Wang, T.; Yu, Z.; Hoon, D. L.; Phee, C. Y.; Lan, Y.; Lu, Y. J. Am. Chem. Soc. 2016, 138, 265; (p) Yu, Y.-N.; Xu, M.-H. Acta Chim. Sinica 2014, 72, 815. (余月娜, 徐明华, 化学学报, 2014, 72, 815.); (q) Zheng, S.; Jia, L.; Liu, Z.; Jiang, D.; Huang, Y.; Nong, N.; Zhang, Q.; Shi, J. Chin. J. Org. Chem. 2014, 34, 1840. (郑珊, 贾莉, 刘志森, 蒋达洪, 黄艳仙, 农兰平, 张庆, 施继成, 有机化学, 2014, 34, 1840.).
[12] (a) Wang, T.; Yao, W.; Zhong, F.; Pang, G. H.; Lu, Y. Angew. Chem., Int. Ed. 2014, 53, 2964; (b) Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Angew. Chem., Int. Ed. 2011, 50, 7837; (c) Han, X.; Zhong, F.; Wang, Y.; Lu, Y. Angew. Chem., Int. Ed. 2012, 51, 767.
[13] (a) Xia, Y.; Liang, Y.; Chen, Y.; Wang, M.; Jiao, L.; Huang, F.; Liu, S.; Li, Y.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 3470; (b) Huang, G.-T.; Lankau, T.; Yu, C.-H. J. Org. Chem. 2014, 79, 1700. |