化学学报 ›› 2021, Vol. 79 ›› Issue (11): 1376-1384.DOI: 10.6023/A21060266 上一篇 下一篇
研究论文
陈德胜a,b,d, 刘葳豪c, 黄清钢a,b, 曹石巍a,b, 田伟a,b, 殷小杰a,b, 谈存敏a,b, 王洁茹a,b,d,e, 初剑a,d, 贾子萌a,d, 程念炜e, 高瑞勤a,b, 吴晓蕾a,b, 秦芝a,b,*( ), 范芳丽a,b, 白静a,b, 李飞泽c, 廖家莉c, 杨远友c, 刘宁c
), 范芳丽a,b, 白静a,b, 李飞泽c, 廖家莉c, 杨远友c, 刘宁c
投稿日期:2021-06-11
发布日期:2021-08-17
通讯作者:
秦芝
基金资助:
Desheng Chena,b,d, Weihao Liuc, Qinggang Huanga,b, Shiwei Caoa,b, Wei Tiana,b, Xiaojie Yina,b, Cunmin Tana,b, Jieru Wanga,b,d,e, Jian Chua,d, Zimeng Jiaa,d, Nianwei Chenge, Ruiqin Gaoa,b, Xiaolei Wua,b, Zhi Qina,b( ), Fangli Fana,b, Jing Baia,b, Feize Lic, Jiali Liaoc, Yuanyou Yangc, Ning Liuc
), Fangli Fana,b, Jing Baia,b, Feize Lic, Jiali Liaoc, Yuanyou Yangc, Ning Liuc
Received:2021-06-11
Published:2021-08-17
Contact:
Zhi Qin
Supported by:文章分享
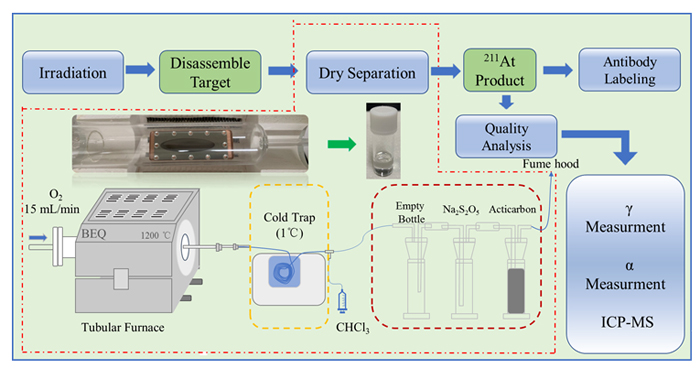
211At, 半衰期7.2 h, 发射的α粒子具有很高的线性传能密度, 是一种理想的靶向α治疗核素. 利用中国科学院近代物理研究所强流超导直线加速器提供的α束流辐照Bi靶生产211At, 系统考察了加速器生产211At的整个流程, 包括加速器辐照、干蒸馏分离、质量分析以及单抗标记. 研究结果表明, 干蒸馏分离211At的总收率最高达78.53%, 获得的固体211At产品具有极高的比活度、核纯度以及化学纯度, 其中杂质元素Bi、Cu、Zn、Al含量均低于100 ng/GBq, 且入射粒子能量低于28.2 MeV时, N(210At)/N(211At)值低于10–5, 研究也实现了211At在尼妥珠单抗上的标记, 标记率高达94.86%. 基于以上研究我们确立了一套简单、高效的分离方法, 为后续我国211At的生产和应用奠定了良好基础.
陈德胜, 刘葳豪, 黄清钢, 曹石巍, 田伟, 殷小杰, 谈存敏, 王洁茹, 初剑, 贾子萌, 程念炜, 高瑞勤, 吴晓蕾, 秦芝, 范芳丽, 白静, 李飞泽, 廖家莉, 杨远友, 刘宁. 加速器生产医用同位素211At及单抗标记[J]. 化学学报, 2021, 79(11): 1376-1384.
Desheng Chen, Weihao Liu, Qinggang Huang, Shiwei Cao, Wei Tian, Xiaojie Yin, Cunmin Tan, Jieru Wang, Jian Chu, Zimeng Jia, Nianwei Cheng, Ruiqin Gao, Xiaolei Wu, Zhi Qin, Fangli Fan, Jing Bai, Feize Li, Jiali Liao, Yuanyou Yang, Ning Liu. Accelerator Production of the Medical Isotope 211At and Monoclonal Antibody Labeling[J]. Acta Chimica Sinica, 2021, 79(11): 1376-1384.
| 靶材料 | Bi-209 | Bi-209 | Bi-209 | Bi-209 |
|---|---|---|---|---|
| 束流能量(MeV) | 30.10 | 30.65 | 29.90 | 29.60 |
| 打靶数量 | 7 | 1 | 2 | 1 |
| 能损a(MeV)/碳膜窗(40 μm) | 1.42 | 1.40 | 1.42 | 1.42 |
| 能损a(MeV)/冷却氦气 1.25 atm (65 mm) | 0.34 | 0.33 | 0b | 0a |
| 剩余能量(MeV) | 28.34 | 28.92 | 28.48 | 28.18 |
| 束流与靶面夹角(°) | 15 | 15 | 15 | 15 |
| 靶材料 | Bi-209 | Bi-209 | Bi-209 | Bi-209 |
|---|---|---|---|---|
| 束流能量(MeV) | 30.10 | 30.65 | 29.90 | 29.60 |
| 打靶数量 | 7 | 1 | 2 | 1 |
| 能损a(MeV)/碳膜窗(40 μm) | 1.42 | 1.40 | 1.42 | 1.42 |
| 能损a(MeV)/冷却氦气 1.25 atm (65 mm) | 0.34 | 0.33 | 0b | 0a |
| 剩余能量(MeV) | 28.34 | 28.92 | 28.48 | 28.18 |
| 束流与靶面夹角(°) | 15 | 15 | 15 | 15 |
| 序号 | 靶厚/(mg•cm–2) | 入射能量/MeV | 平均流强/μA | 辐照时间/h | EOB活度/MBq | |
|---|---|---|---|---|---|---|
| 理论值 | 实测值 | |||||
| 1 | 10.18 | 28.34 | 1.88 | 1.20 | 45.97 | 43.83±4.26 |
| 2 | 13.83 | 28.34 | 2.03 | 1.03 | 50.57 | 41.98±3.62 |
| 3 | 15.71 | 28.34 | 1.90 | 1.13 | 54.11 | 49.49±2.88 |
| 4 | 17.48 | 28.34 | 1.95 | 2.05 | 103.78 | 118.82±7.08 |
| 5 | 19.89 | 28.34 | 2.05 | 2.01 | 107.78 | 61.78±3.63 |
| 6 | 15.43 | 28.34 | 1.85 | 2.26 | 105.69 | 109.89±6.34 |
| 7 | 9.97 | 28.34 | 2.07 | 2.04 | 83.91 | 85.97±4.96 |
| 8 | 10.18 | 28.92 | 2.05 | 1.50 | 76.31 | 88.83±5.16 |
| 9 | 19.07 | 28.48 | 7.07 | 1.83 | 344.97 | 73.36±1.81 |
| 10 | 18.55 | 28.48 | 8.18 | 4.08 | 889.78 | 314.61±3.47 |
| 11 | 19.05 | 28.18 | 10.02 | 4.08 | 993.87 | 351.18±4.12 |
| 序号 | 靶厚/(mg•cm–2) | 入射能量/MeV | 平均流强/μA | 辐照时间/h | EOB活度/MBq | |
|---|---|---|---|---|---|---|
| 理论值 | 实测值 | |||||
| 1 | 10.18 | 28.34 | 1.88 | 1.20 | 45.97 | 43.83±4.26 |
| 2 | 13.83 | 28.34 | 2.03 | 1.03 | 50.57 | 41.98±3.62 |
| 3 | 15.71 | 28.34 | 1.90 | 1.13 | 54.11 | 49.49±2.88 |
| 4 | 17.48 | 28.34 | 1.95 | 2.05 | 103.78 | 118.82±7.08 |
| 5 | 19.89 | 28.34 | 2.05 | 2.01 | 107.78 | 61.78±3.63 |
| 6 | 15.43 | 28.34 | 1.85 | 2.26 | 105.69 | 109.89±6.34 |
| 7 | 9.97 | 28.34 | 2.07 | 2.04 | 83.91 | 85.97±4.96 |
| 8 | 10.18 | 28.92 | 2.05 | 1.50 | 76.31 | 88.83±5.16 |
| 9 | 19.07 | 28.48 | 7.07 | 1.83 | 344.97 | 73.36±1.81 |
| 10 | 18.55 | 28.48 | 8.18 | 4.08 | 889.78 | 314.61±3.47 |
| 11 | 19.05 | 28.18 | 10.02 | 4.08 | 993.87 | 351.18±4.12 |

| 靶序号 | 温度/℃ | 气流量/(mL•min–1) | 保温时间/min | 洗涤液 | 洗涤液用量(mL) | 收集率 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 一段 | 二段 | 三段 | 一段 | 二段 | 三段 | ||||||
| 1 | 850 | 15 | 15 | CHCl3 | 0.4 | 2.0 | — | — | — | — | |
| 2 | 850 | 15 | 15 | CHCl3 | 0.4 | 1.0 | — | — | — | — | |
| 3 | 850 | 15 | 15 | CHCl3 | 5.0 | 10.0 | — | — | — | — | |
| 4 | 850 | 15 | 15 | CHCl3 | 4.0 | 5.0 | — | 54.85±8.22% | 1.42±8.35% | — | |
| 5 | 850 | 08 | 15 | CHCl3 | 1.0 | 5.0 | — | 34.40±8.29% | 3.51±8.28% | — | |
| 6 | 850 | 10 | 15 | CHCl3 | 1.0 | 5.0 | — | 27.73±8.17% | 2.48±8.17% | — | |
| 7 | 850 | 15 | 15 | C2H6O | 0.4 | 1.0 | — | 39.45±8.15% | 5.14±8.17% | — | |
| 8 | 850 | 15 | 25 | C2H6O | 0.8 | 1.0 | — | 41.63±8.22% | 3.37±8.29% | — | |
| 9 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | — | 42.83±5.60% | 4.53±5.93% | — | |
| 10 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | — | 44.71±5.82% | 17.44±6.30% | — | |
| 11 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | 1.0 | 48.69±5.79% | 15.86±6.36% | 13.98±6.43% | |
| 靶序号 | 温度/℃ | 气流量/(mL•min–1) | 保温时间/min | 洗涤液 | 洗涤液用量(mL) | 收集率 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 一段 | 二段 | 三段 | 一段 | 二段 | 三段 | ||||||
| 1 | 850 | 15 | 15 | CHCl3 | 0.4 | 2.0 | — | — | — | — | |
| 2 | 850 | 15 | 15 | CHCl3 | 0.4 | 1.0 | — | — | — | — | |
| 3 | 850 | 15 | 15 | CHCl3 | 5.0 | 10.0 | — | — | — | — | |
| 4 | 850 | 15 | 15 | CHCl3 | 4.0 | 5.0 | — | 54.85±8.22% | 1.42±8.35% | — | |
| 5 | 850 | 08 | 15 | CHCl3 | 1.0 | 5.0 | — | 34.40±8.29% | 3.51±8.28% | — | |
| 6 | 850 | 10 | 15 | CHCl3 | 1.0 | 5.0 | — | 27.73±8.17% | 2.48±8.17% | — | |
| 7 | 850 | 15 | 15 | C2H6O | 0.4 | 1.0 | — | 39.45±8.15% | 5.14±8.17% | — | |
| 8 | 850 | 15 | 25 | C2H6O | 0.8 | 1.0 | — | 41.63±8.22% | 3.37±8.29% | — | |
| 9 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | — | 42.83±5.60% | 4.53±5.93% | — | |
| 10 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | — | 44.71±5.82% | 17.44±6.30% | — | |
| 11 | 850 | 15 | 20 | CHCl3 | 0.4 | 0.4 | 1.0 | 48.69±5.79% | 15.86±6.36% | 13.98±6.43% | |
| 种态 | At | AtO2 | HAtO |
|---|---|---|---|
| –ΔHads/(kJ•mol–1) | 124±5 | 83±5 | 47±5 |
| ΔHsubl/(kJ•mol–1) | 148±25 | 106±30 | 56±30 |
| 种态 | At | AtO2 | HAtO |
|---|---|---|---|
| –ΔHads/(kJ•mol–1) | 124±5 | 83±5 | 47±5 |
| ΔHsubl/(kJ•mol–1) | 148±25 | 106±30 | 56±30 |
| 能量(MeV) | N(210At)/N(211At) | |
|---|---|---|
| α能谱法 | γ能谱法 | |
| 28.18 | 7.07×10–6 | <5.73×10–7 |
| 28.34 | 1.61×10–5 | 4.19×10–6 |
| 28.48 | 4.35×10–5 | 1.42×10–5 |
| 28.92 | 3.05×10–4 | 1.38×10–4 |
| 能量(MeV) | N(210At)/N(211At) | |
|---|---|---|
| α能谱法 | γ能谱法 | |
| 28.18 | 7.07×10–6 | <5.73×10–7 |
| 28.34 | 1.61×10–5 | 4.19×10–6 |
| 28.48 | 4.35×10–5 | 1.42×10–5 |
| 28.92 | 3.05×10–4 | 1.38×10–4 |
| t/h | PBS/% | FBS/% |
|---|---|---|
| 3 | 94.25 | 87.34 |
| 6 | 92.73 | 86.42 |
| 12 | 85.71 | 76.71 |
| 24 | 79.31 | 73.87 |
| t/h | PBS/% | FBS/% |
|---|---|---|
| 3 | 94.25 | 87.34 |
| 6 | 92.73 | 86.42 |
| 12 | 85.71 | 76.71 |
| 24 | 79.31 | 73.87 |
| [1] |
Nefedov V. D.; Norseev Y. V.; Toropova M. A.; Khalkin V. A. Russ. Chem. Rev. 1968, 37, 193.
|
| [2] |
Corson D. R.; Mackenzie K. R.; Segrè E. J. Phys. Rev. 1940, 58.
|
| [3] |
Yanokura M.; Kudo H.; Nakahara H.; Miyano K.; Ohya S.; Nitoh O. Nucl. Phys. A 1978, 299, 92.
doi: 10.1016/0375-9474(78)90210-5 |
| [4] |
Bäck T.,Alpha-radioimmunotherapy with At-211: Evaluations and Imaging on Normal Tissues and Tumors, University of Gothenburg, Gothenburg, 2011, pp. 8-12.
|
| [5] |
Bakr H. H. M.S. Thesis, University of Gothenburg, Gothenburg, 2014.
|
| [6] |
Brown I., Int. J. Radiat. Appl. Instrum. Part A 1986, 37, 789.
doi: 10.1016/0883-2889(86)90273-X |
| [7] |
Morzenti S.; Bonardi M. L.; Groppi F.; Zona C.; Persico E.; Menapace E.; Alfassi Z. B. J. Radioanal. Nucl. Chem. 2008, 276, 843.
doi: 10.1007/s10967-008-0642-6 |
| [8] |
Gagnon K.; Risler R.; Pal S.; Hamlin D.; Orzechowski J.; Pavan R.; Zeisler S.; Wilbur D. S. J. Labelled Compd. Radiopharm. 2012, 55, 436.
doi: 10.1002/jlcr.2968 |
| [9] |
Bochvarova M.; Do Kim T.; Dudova I.; Norseev Y. V.; Khalkin V. Radiokhimiya 1972, 14, 858.
|
| [10] |
Wang F. C.; Kang M. H.; Khalkin V. A. Radiokhimiya 1962, 4, 94.
|
| [11] |
Meyer G.-J.; Lambrecht R. J. Labelled Compd. Radiopharm. 1981, 18, 233.
|
| [12] |
Meyer G.; Lambrecht R. Int. J. Appl. Radiat. Isot. 1980, 31, 351.
doi: 10.1016/0020-708X(80)90125-8 |
| [13] |
Dasgupta M.; Hinde D.; Hagino K.; Moraes S.; Gomes P.; Anjos R.; Butt R.; Berriman A.; Carlin N.; Morton C. Phys. Rev. C 2002, 66, 041602.
doi: 10.1103/PhysRevC.66.041602 |
| [14] |
Lindegren S.; Bäck T.; Jensen H. J. Appl. Radiat. Isot. 2001, 55, 157.
doi: 10.1016/S0969-8043(01)00044-6 |
| [15] |
Martin T. M., Ph.D. Dissertation, Texas A&M University, College Station, 2018.
|
| [16] |
Wang Y.; Sato N.; Komori Y.; Yokokita T.; Mori D.; Usuda S.; Haba H. RIKEN Accel. Prog. Rep, 2017, 50, 262.
|
| [17] |
Zona C.; Bonardi M. L.; Groppi F.; Morzenti S.; Canella L.; Persico E.; Menapace E.; Alfassi Z. B.; Abbas K.; Holzwarth U. J. Radioanal. Nucl. Chem. 2008, 276, 819.
doi: 10.1007/s10967-008-0638-2 |
| [18] |
Yordanov A.; Pozzi O.; Carlin S.; Akabani G.; Wieland B.; Zalutsky M. J. Radioanal. Nucl. Chem. 2004, 262, 593.
doi: 10.1007/s10967-005-0481-7 |
| [19] |
O’hara M. J.; Krzysko A. J.; Niver C. M.; Morrison S. S.; Owsley S. L.; Hamlin D. K.; Dorman E. F.; Scott Wilbur D. Appl. Radiat. Isot. 2017, 122, 202.
doi: 10.1016/j.apradiso.2017.02.001 |
| [20] |
Sundberg Å. L.; Almqvist Y.; Tolmachev V.; Carlsson J. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 727.
doi: 10.1007/s00259-003-1129-x |
| [21] |
Willhauck M. J.; Samani B.-R. S.; Wolf I.; Senekowitsch- Schmidtke R.; Stark H.-J.; Meyer G. J.; Knapp W. H.; Göke B.; Morris J. C.; Spitzweg C. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1272.
doi: 10.1007/s00259-008-0775-4 |
| [22] |
Petrich T.; Korkmaz Z.; Krull D.; Frömke C.; Meyer G. J.; Knapp W. H. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 851.
doi: 10.1007/s00259-009-1356-x |
| [23] |
Robinson M. K.; Shaller C.; Garmestani K.; Plascjak P. S.; Hodge K. M.; Yuan Q.-A.; Marks J. D.; Waldmann T. A.; Brechbiel M. W.; Adams G. P. Clin. Cancer Res. 2008, 14, 875.
doi: 10.1158/1078-0432.CCR-07-1250 pmid: 18245551 |
| [24] |
Aurlien E.; Larsen R.; Kvalheim G.; Bruland O. Br. J. Cancer 2000, 83, 1375.
doi: 10.1054/bjoc.2000.1453 |
| [25] |
Andersson H.; Elgqvist J.; Horvath G.; Hultborn R.; Jacobsson L.; Jensen H.; Karlsson B.; Lindegren S.; Palm S. Clin. Cancer Res. 2003, 9, 3914s.
|
| [26] |
Zalutsky M. R.; Pruszynski M. Curr. Radiopharm. 2011, 4, 177.
pmid: 22201707 |
| [27] |
Makvandi M.; Dupis E.; Engle J. W.; Nortier F. M.; Fassbender M. E.; Simon S.; Birnbaum E. R.; Atcher R. W.; John K. D.; Rixe O. Targeted Oncol. 2018, 13, 189.
doi: 10.1007/s11523-018-0550-9 |
| [28] |
Kiess A.; Minn I.; Vaidyanathan G.; Hobbs R. F.; Josefsson A.; Shen C.; Brummet M.; Chen Y.; Choi J.; Koumarianou E. J. Nucl. Med. 2016, 57, 1569.
doi: 10.2967/jnumed.116.174300 |
| [29] |
Dziawer L.; Komiński P.; Mczyńska-Wielgosz S.; Pruszyński M.; Yczko M.; Ws B.; Celichowski G.; Grobelny J.; Jastrzbski J.; Bilewicz A. RSC Adv. 2017, 7, 41024.
doi: 10.1039/C7RA06376H |
| [30] |
Zalutsky M. R.; Reardon D. A.; Akabani G.; Coleman R. E.; Friedman A. H.; Friedman H. S.; Mclendon R. E.; Wong T. Z.; Bigner D. D. J. Nucl. Med. 2008, 49, 30.
doi: 10.2967/jnumed.107.046938 |
| [31] |
Andersson H.; Cederkrantz E.; Bäck T.; Divgi C.; Elgqvist J.; Himmelman J.; Horvath G.; Jacobsson L.; Jensen H.; Lindegren S. J. Nucl. Med. 2009, 50, 1153.
doi: 10.2967/jnumed.109.062604 |
| [32] |
Liu N.; Jin J.; Zhang S.; Mo S.; Yang Y.; Wang J.; Zhou M. J. Radioanal. Nucl. Chem. 2001, 247, 129.
doi: 10.1023/A:1006727401721 |
| [33] |
Liu N.; Jin J.; Mo S.; Chen H.; Yu Y. J. Radioanal. Nucl. Chem. 1998, 227, 187.
doi: 10.1007/BF02386459 |
| [34] |
Liu N.; Jin J.; Zhang S.; Luo D.; Wang J.; Zhou M.; Luo L.; Wang F. J. Labelled Compd. Radiopharm. 1995, 36, 1105.
doi: 10.1002/jlcr.v36:11 |
| [35] |
Tarasov O. B.; Bazin D. Nucl. Instrum. Methods Phys. Res. 2008, 266, 4657.
doi: 10.1016/j.nimb.2008.05.110 |
| [36] |
http://lise.nscl.msu.edu/lise.html.
|
| [37] |
Otuka N.; Takács S. Radiochim. Acta 2015, 103, 1.
doi: 10.1515/ract-2013-2234 |
| [38] |
Serov A.; Aksenov N.; Bozhikov G.; Eichler R.; Dressler R.; Lebedev V. Y.; Petrushkin O.; Piguet D.; Shishkin S.; Tereshatov E. Radiochim. Acta 2011, 99, 593.
doi: 10.1524/ract.2011.1850 |
| [39] |
Shinohara A.; Toyoshima A.; Yoshimura T.; Kanda A.CN 201880078835. 5, 2020.
|
|
( 篠原厚, 丰岛厚史, 吉村崇, 神田晃充,CN 201880078835. 5, 2020.)
|
|
| [40] |
Demidov Y.; Zaitsevskii A. Chem. Phys. Lett. 2018, 691, 126.
doi: 10.1016/j.cplett.2017.11.008 |
| [41] |
Sun R.; Kang X.; Wang L.; Xu Y. J. Univ. South China (Sci. Technol.), 2017, 31, 37. (in Chinese)
|
|
( 孙荣忠, 康玺, 王郦彬, 许艳婷, 南华大学学报(自然科学版), 2017, 31, 37.)
|
|
| [42] |
Vennart J. J. Radiol. Prot. 1991, 11, 199.
doi: 10.1088/0952-4746/11/3/006 |
| [43] |
Liu W.; Ma H.; Tang Y.; Chen Q.; Peng S.; Yang J.; Liao J.; Yang Y.; Li Q.; Liu N. J. Radioanal. Nucl. Chem. 2018, 316, 451.
doi: 10.1007/s10967-018-5780-x |
| [44] |
Liu N.; Jin J.; Zhang S.; Mo Shang.; Yang Y.; Wang J.; Zhou M.; J. Radioanal. Nucl. Chem. 2001, 247, 129.
doi: 10.1023/A:1006727401721 |
| [45] |
Ning L.; Yang Y.; Zan L.; Liao J.; Jin J. J. Radioanal. Nucl. Chem. 2007, 272, 85.
doi: 10.1007/s10967-006-6781-8 |
| [46] |
Yang Y.; Lin R.; Ning L.; Liao J.; Jin J. J. Radioanal. Nucl. Chem. 2010, 288.
|
| [47] |
Glockler G. J. Phys. Chem. 1959, 63, 828.
doi: 10.1021/j150576a013 |
| [48] |
Lin J. L.; Bent B. E. J. Phys. Chem. 1992, 96, 8529.
doi: 10.1021/j100200a059 |
| [49] |
Li J. L., Laboratory γ Measurement and Analysis of Lenergy Spectrum, China Communications Press, Beijing, 2014, pp. 88-93. (in Chinese)
|
|
( 李君利, 实验室γ能谱测量与分析, 人民交通出版社, 北京, 2014, pp. 88-93.)
|
|
| [50] |
Zvara I. J. Radioanal. Nucl. Chem. 1996, 204, 123.
doi: 10.1007/BF02060873 |
| [51] |
Zvára I., The Inorganic Radiochemistry of Heavy Elements: Methods for Studying Gaseous Compounds, Springer Science & Business Media, Heidelberg, 2008, pp. 87-118.
|
| [52] |
Zvára I. Radiochim. Acta 1985, 38, 95.
doi: 10.1524/ract.1985.38.2.95 |
| [53] |
Lin M.; Qin Z.; Guo J.; Zhang L.; Ding H.; Fan F.; Bai J.; Lei F.; Wu X.; Li X. J. Nucl. Radiochem. 2009, 31, 28. (in Chinese)
|
|
( 林茂盛, 秦芝, 郭俊盛, 张丽娜, 丁华杰, 范芳丽, 白静, 雷富安, 吴晓蕾, 李小飞, 核化学与放射化学, 2009, 31, 28.)
|
| [1] | 邱玲, 梁家艺, 张竹霞, 王太山. 15N同位素标记的金属氮化物内嵌富勒烯的合成与表征[J]. 化学学报, 2022, 80(7): 874-878. |
| [2] | 陈玉宛, 周雯, 李欣蔚, 杨开广, 梁振, 张丽华, 张玉奎. 基于液质联用技术的蛋白质-蛋白质相互作用研究进展※[J]. 化学学报, 2022, 80(6): 817-826. |
| [3] | 夏雷, 程震, 朱华, 杨志. 124I原位标记有机黑色素纳米粒子的制备及初步分子影像研究[J]. 化学学报, 2019, 77(2): 172-178. |
| [4] | 杨麦云, 陈鹏. 生物正交标记反应研究进展[J]. 化学学报, 2015, 73(8): 783-792. |
| [5] | 薛向东, 孙玉姣, 刘洋, 张萍, 王仲孚, 黄琳娟. 基于电喷雾电离质谱(ESI-MS)的丙酮稳定同位素标记对N-糖链的相对定量分析方法的研究[J]. 化学学报, 2014, 72(2): 220-226. |
| [6] | 林洁华, 张慧慧, 邵美佳. 基于离子液体修饰介孔硅的免标记电化学免疫测定双组分肿瘤标志物[J]. 化学学报, 2014, 72(2): 241-245. |
| [7] | 姜涛, 杨君, 杨亮, 何玉晖. 加速器质谱测量用NH4Hf2F9 的制备及表征[J]. 化学学报, 2012, 70(9): 1135-1138. |
| [8] | 邓洪平, 王国建, 朱邦尚, 朱利娟, 王大力, 庄园园, 朱新远. 基于共轭高分子复合物能量转移的非标记DNA检测[J]. 化学学报, 2012, 70(24): 2507-2512. |
| [9] | 王玥, 叶新山. 基于Diels-Alder生物正交反应的蛋白质快速位点特异性标记方法[J]. 化学学报, 2012, 70(21): 2208-2212. |
| [10] | 李劼, 王杰, 陈鹏. 基于非天然氨基酸的蛋白质生物正交标记[J]. 化学学报, 2012, 70(13): 1439-1445. |
| [11] | 上官莉, 漆红兰, 凌晨. 非标记夹心式电化学可卡因适体传感器的研究[J]. 化学学报, 2011, 69(18): 2196-2200. |
| [12] | 关松磊, 吕磊, 胡秀丽, 刘忠英, 宋凤瑞, 刘志强. 顺铂对卵巢癌COC1细胞总蛋白表达的影响研究[J]. 化学学报, 2011, 69(14): 1721-1724. |
| [13] | 刘训悦, 阚登蕾, 丁兴成. 新型杀菌剂唑菌酯吡唑环14C标记合成和鉴定[J]. 化学学报, 2011, 69(12): 1445-1449. |
| [14] | 王潇蕤, 李继睿. 金增强多重胶体金标记树枝状复合物放大的电化学免疫分析新方法[J]. 化学学报, 2011, 69(10): 1211-1216. |
| [15] | 张治红, 梁燕, 闫福丰, 闫立军, 豆君, 李彦山, 王力臻, 郑先君. 无标记DNA在氨基改性导电聚吡咯表面的固定/杂交[J]. 化学学报, 2010, 68(9): 833-838. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||