化学学报 ›› 2022, Vol. 80 ›› Issue (10): 1376-1384.DOI: 10.6023/A22070288 上一篇 下一篇
研究论文
殷东a, 商宏怡a, 余文浩a,b, 向仕凯a, 胡平a, 赵可清a, 冯春a,*( ), 汪必琴a,*(
), 汪必琴a,*( )
)
投稿日期:2022-07-01
发布日期:2022-09-06
通讯作者:
冯春, 汪必琴
基金资助:
Dong Yina, Hongyi Shanga, Wenhao Yua,b, Shikai Xianga, Ping Hua, Keqing Zhaoa, Chun Fenga( ), Biqin Wanga(
), Biqin Wanga( )
)
Received:2022-07-01
Published:2022-09-06
Contact:
Chun Feng, Biqin Wang
Supported by:文章分享

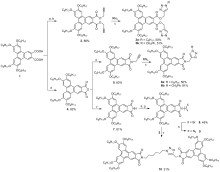
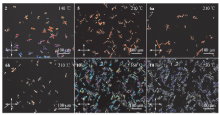
在盘状液晶分子外围柔性链中引入功能不同的基团, 是设计和合成具有良好液晶性能化合物的常用策略. 本工作从苯并菲2,3-二甲酸和酸酐出发, 通过亲核取代和点击反应, 分别合成了两类三唑环修饰的苯并菲二羧酸酯和苯并菲酰亚胺化合物. 热重分析仪(TGA)测试表明, 新制备的主要中间产物与目标化合物失重为5%时的温度在274~389 ℃之间, 均表现出良好的热稳定性. 我们通过差示扫描量热法(DSC)、偏光显微镜(POM)和变温X射线衍射(XRD)实验对这些化合物的热力学行为和介晶性进行了研究. 除三唑环修饰的酯3a和3b无液晶性外, 主要中间产物(2和5)与三唑环修饰的酰亚胺产物(6a和6b)均有一个六方柱状液晶相. 此外, 制备的以三唑为桥联基团的酰亚胺二聚体10为室温液晶, 具有173 ℃的液晶范围. XRD证实了该二聚体中存在有序度不同的两种柱状液晶相(Colh1和Colh2). 另外, 由于三唑环的引入, 三唑环修饰的酯类和酰亚胺两类化合物在某些有机溶剂中都能形成有机凝胶. 其中, 二聚体10在1,2-二氯乙烷和1,4-二氧六环中表现出优异的凝胶能力, 其临界凝胶浓度(CGC)可低至1 mg/mL. 相比之下, 不含三唑环的中间产物2和5则不能形成凝胶, 表明三唑环之间的偶极-偶极作用和液晶刚性核π-π堆积的协同作用对于超分子凝胶的形成起着重要的作用. 因此, 同时表现出液晶和凝胶性质的三唑环修饰的苯并菲2,3-二酰亚胺分子具有成为一种多功能材料的潜在应用价值.
殷东, 商宏怡, 余文浩, 向仕凯, 胡平, 赵可清, 冯春, 汪必琴. 三唑环修饰的苯并菲二羧酸酯和酰亚胺: 合成、液晶及凝胶性[J]. 化学学报, 2022, 80(10): 1376-1384.
Dong Yin, Hongyi Shang, Wenhao Yu, Shikai Xiang, Ping Hu, Keqing Zhao, Chun Feng, Biqin Wang. Synthesis, Mesomorphism and Gelation Properties of Triazole-Modified Triphenylene 2,3-Dicarboxylic Esters and 2,3-Dicarboxyimides[J]. Acta Chimica Sinica, 2022, 80(10): 1376-1384.


| Compd. | Mesophases, transition temperature and enthalpy changes | |
|---|---|---|
| 2nd heating/℃ (ΔH/(kJ•mol–1)) | 1st cooling/℃ (ΔH/(kJ•mol–1)) | |
| 2 | Cr 77 (45.8) Colh 142 (5.3) I | I 137 (5.0) Colh 5 (19.2) Cr |
| 5 | Cr 130 (55.5) Colh 224 (4.3) I | I 220 (4.9) Colh 84 (51.5) Cr |
| 3a | Cr 118 (33.6) I | I 101 (43.2) Cr |
| 3b | Cr 159 (41.6) I | I 123 (15.6) Cr |
| 6a | Cr 108 (36.6) Colh 215 (6.7) I | I 212 (5.6) Colh 63 (39.6) Cr |
| 6b | Cr 165 (31.2) Colh 223 (6.4) I | I 221 (7.4) Colh 88 (27.7) Cr |
| 10 | Cr 21 (2.4) Colh1 161 (1.6) Colh2187 (3.1) I | I 181 (4.3) Colh2 136 (1.2) Colh1 8 (2.4) Cr |
| Compd. | Mesophases, transition temperature and enthalpy changes | |
|---|---|---|
| 2nd heating/℃ (ΔH/(kJ•mol–1)) | 1st cooling/℃ (ΔH/(kJ•mol–1)) | |
| 2 | Cr 77 (45.8) Colh 142 (5.3) I | I 137 (5.0) Colh 5 (19.2) Cr |
| 5 | Cr 130 (55.5) Colh 224 (4.3) I | I 220 (4.9) Colh 84 (51.5) Cr |
| 3a | Cr 118 (33.6) I | I 101 (43.2) Cr |
| 3b | Cr 159 (41.6) I | I 123 (15.6) Cr |
| 6a | Cr 108 (36.6) Colh 215 (6.7) I | I 212 (5.6) Colh 63 (39.6) Cr |
| 6b | Cr 165 (31.2) Colh 223 (6.4) I | I 221 (7.4) Colh 88 (27.7) Cr |
| 10 | Cr 21 (2.4) Colh1 161 (1.6) Colh2187 (3.1) I | I 181 (4.3) Colh2 136 (1.2) Colh1 8 (2.4) Cr |
| Compd. | Temperature (Mesophase) | 2θ/(°) | dobs/nm | dcalcd/nm | I/% | hkl | Lattice parameters |
|---|---|---|---|---|---|---|---|
| 2 | 200 ℃ (Colh) | 4.87 19.73 25.13 | 1.81 0.45 0.35 | — | 100 0.1 0.1 | 100 hch hπ | a=2.09 nm A=3.78 nm2 Z=0.9 |
| 5 | 150 ℃ (Colh) | 4.89 19.76 25.83 | 1.81 0.45 0.35 | — | 100 0.1 22.1 | 100 hch hπ | a=2.08 nm |
| A=3.75 nm2 | |||||||
| Z=0.9 | |||||||
| 6a | 140 ℃ (Colh) | 4.28 19.94 25.34 | 2.06 0.44 0.35 | — | 100 0.1 18.8 | 100 hch hπ | a=2.38 nm A=4.91 nm2 Z=1.0 |
| 6b | 140 ℃ (Colh) | 4.46 19.73 25.6 | 1.98 0.45 0.35 | — | 100 1.0 10.5 | 100 hch hπ | a=2.29 nm A=4.54 nm2 Z=1.0 |
| 10 | 170 ℃ (Colh2) | 4.39 19.68 25.03 | 2.01 0.45 0.35 | — | 100 0.4 29.5 | 100 hch hπ | a=2.32 nm A=4.66 nm2 Z=0.5 |
| 60 ℃ (Colh1) | 4.48 10.12 12.66 15.52 20.95 23.13 25.66 | 1.97 0.87 0.70 0.57 0.42 0.38 0.35 | 1.97 0.85 0.68 0.57 — 0.38 — | 9.7 0.1 0.2 0.2 0.2 0.7 100 | 110 400 500 600 hch 730 hπ | a=3.94 nm A=13.444 nm2 Z=1.4 |
| Compd. | Temperature (Mesophase) | 2θ/(°) | dobs/nm | dcalcd/nm | I/% | hkl | Lattice parameters |
|---|---|---|---|---|---|---|---|
| 2 | 200 ℃ (Colh) | 4.87 19.73 25.13 | 1.81 0.45 0.35 | — | 100 0.1 0.1 | 100 hch hπ | a=2.09 nm A=3.78 nm2 Z=0.9 |
| 5 | 150 ℃ (Colh) | 4.89 19.76 25.83 | 1.81 0.45 0.35 | — | 100 0.1 22.1 | 100 hch hπ | a=2.08 nm |
| A=3.75 nm2 | |||||||
| Z=0.9 | |||||||
| 6a | 140 ℃ (Colh) | 4.28 19.94 25.34 | 2.06 0.44 0.35 | — | 100 0.1 18.8 | 100 hch hπ | a=2.38 nm A=4.91 nm2 Z=1.0 |
| 6b | 140 ℃ (Colh) | 4.46 19.73 25.6 | 1.98 0.45 0.35 | — | 100 1.0 10.5 | 100 hch hπ | a=2.29 nm A=4.54 nm2 Z=1.0 |
| 10 | 170 ℃ (Colh2) | 4.39 19.68 25.03 | 2.01 0.45 0.35 | — | 100 0.4 29.5 | 100 hch hπ | a=2.32 nm A=4.66 nm2 Z=0.5 |
| 60 ℃ (Colh1) | 4.48 10.12 12.66 15.52 20.95 23.13 25.66 | 1.97 0.87 0.70 0.57 0.42 0.38 0.35 | 1.97 0.85 0.68 0.57 — 0.38 — | 9.7 0.1 0.2 0.2 0.2 0.7 100 | 110 400 500 600 hch 730 hπ | a=3.94 nm A=13.444 nm2 Z=1.4 |


| Compd. | Solvent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Hex | DMF | THF | DCE | EA | Methanol | Acetonitrile | 1,4-Dioxane | |
| 3a | S | I | S | S | S | S | G (1) | G (2) | I |
| 3b | S | I | S | S | S | S | G (2) | G (1) | I |
| 6a | S | G (4) | G (4) | S | S | PS | I | I | PS |
| 6b | G (4) | G (2) | G (4) | S | S | G (4) | I | I | PS |
| 10 | S | PS | S | S | G (1) | PS | I | I | G (1) |
| Compd. | Solvent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Hex | DMF | THF | DCE | EA | Methanol | Acetonitrile | 1,4-Dioxane | |
| 3a | S | I | S | S | S | S | G (1) | G (2) | I |
| 3b | S | I | S | S | S | S | G (2) | G (1) | I |
| 6a | S | G (4) | G (4) | S | S | PS | I | I | PS |
| 6b | G (4) | G (2) | G (4) | S | S | G (4) | I | I | PS |
| 10 | S | PS | S | S | G (1) | PS | I | I | G (1) |


| [1] |
(a) Wohrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J. C.; Staffeld, P.; Baro, A.; Giesselmann, F.; Laschat, S. Chem. Rev. 2016, 116, 1139.
doi: 10.1021/acs.chemrev.5b00190 pmid: 11498585 |
|
(b) Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; Tosoni, M. Angew. Chem., Int. Ed. 2007, 46, 4832.
doi: 10.1002/anie.200604203 pmid: 11498585 |
|
|
(c) Kumar, S. Liq. Cryst. 2020, 47, 1195.
doi: 10.1080/02678292.2020.1720840 pmid: 11498585 |
|
|
(d) Pal, S. K.; Setia, S.; Avinash, B. S.; Kumar, S. Liq. Cryst. 2013, 40, 1769.
doi: 10.1080/02678292.2013.854418 pmid: 11498585 |
|
|
(e) Kumar, M.; Varshney, S.; Kumar, S. Polym. J. 2021, 53, 283.
pmid: 11498585 |
|
|
(f) Kumar, S. Liq. Cryst. 2004, 31, 1037.
doi: 10.1080/02678290410001724746 pmid: 11498585 |
|
|
(g) Schmidt-Mende, L.; Fechtenkotter, A.; Mullen, K.; Moons, E.; Friend, R. H.; MacKenzie, J. D. Science 2001, 293, 1119.
pmid: 11498585 |
|
|
(h) Zhu, X. M.; Bai, X. Y.; Wang, H. F.; Hu, P.; Wang, B. Q.; Zhao, K. Q. Acta Chim. Sinica 2021, 79, 1486. (in Chinese)
doi: 10.6023/A21080397 pmid: 11498585 |
|
|
(朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清, 化学学报, 2021, 79, 1486.)
doi: 10.6023/A21080397 pmid: 11498585 |
|
|
(i) Chen, S. S.; Li, T.; Zhao, D. H. Acta Phys. Chim. Sin. 2010, 26, 1124. (in Chinese)
doi: 10.3866/PKU.WHXB20100412 pmid: 11498585 |
|
|
(陈树森, 李田, 赵达慧, 物理化学学报, 2010, 26, 1124.)
pmid: 11498585 |
|
| [2] |
(a) Zhao, K. Q.; Wang, B. Q.; Hu, P.; Gao, C. Y.; Yuan, F. J.; Li, H. R. Chin. J. Chem. 2006, 24, 210.
doi: 10.1002/cjoc.200690040 |
|
(b) Zhao, K. Q.; Hu, P.; Wang, B. Q.; Yu, W. H.; Chen, H. M.; Wang, X. L.; Shimizu, Y. Chin. J. Chem. 2007, 25, 375.
doi: 10.1002/cjoc.200790072 |
|
| [3] |
Allen, M. T.; Diele, S.; Harris, K. D. M.; Hegmann, T.; Kariuki, B. M.; Lose, D.; Preece, J. A.; Tschierske, C. J. Mater. Chem. 2001, 11, 302.
doi: 10.1039/b006916g |
| [4] |
Cammidge, A. N.; Chausson, C.; Gopee, H.; Li, J.; Hughes, D. L. Chem. Commun. 2009, 7375.
|
| [5] |
(a) Lavigueur, C.; Foster, E. J.; Williams, V. E. J. Am. Chem. Soc. 2008, 130, 11791.
doi: 10.1021/ja803406k |
|
(b) Voisin, E.; Johan Foster, E.; Rakotomalala, M.; Williams, V. E. Chem. Mater. 2009, 21, 3251.
|
|
| [6] |
Osawa, T.; Kajitani, T.; Hashizume, D.; Ohsumi, H.; Sasaki, S.; Takata, M.; Koizumi, Y.; Saeki, A.; Seki, S.; Fukushima, T. Angew. Chem., Int. Ed. 2012, 51, 7990.
doi: 10.1002/anie.201203077 |
| [7] |
Gao, X.; Hu, Y. J. Mater. Chem. C 2014, 2, 3099.
doi: 10.1039/C3TC32046D |
| [8] |
Gudeika, D. Synth. Met. 2020, 262, 116328.
doi: 10.1016/j.synthmet.2020.116328 |
| [9] |
(a) Wurthner, F.; Saha-Moller, C. R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Chem. Rev. 2016, 116, 962.
doi: 10.1021/acs.chemrev.5b00188 |
|
(b) Hu, Y. H.; Wu, W. L.; Yu, L. Y.; Luo, K. J.; Xu, X. P.; Li, Y.; Peng, Q. Acta Chim. Sinica 2020, 78, 1246. (in Chinese)
doi: 10.6023/A20070282 |
|
|
(胡瑜辉, 武文林, 于立扬, 骆开均, 徐小鹏, 李瑛, 彭强, 化学学报, 2020, 78, 1246.)
doi: 10.6023/A20070282 |
|
| [10] |
Pu, J.; Yang, T.; Wang, Y.; Zhu, Q.; Yang, M.; Liu, C.; Wang, W. Liq. Cryst. 2020, 47, 291.
doi: 10.1080/02678292.2019.1645899 |
| [11] |
(a) Psutka, K. M.; Bozek, K. J. A.; Maly, K. E. Org. Lett. 2014, 16, 5442.
doi: 10.1021/ol502678m pmid: 31362501 |
|
(b) Psutka, K. M.; LeDrew, J.; Taing, H.; Eichhorn, S. H.; Maly, K. E. J. Org. Chem. 2019, 84, 10796.
doi: 10.1021/acs.joc.9b01330 pmid: 31362501 |
|
| [12] |
Yin, J.; Qu, H. M.; Zhang, K.; Luo, J.; Zhang, X. J.; Chi, C. Y.; Wu, J. S. Org. Lett. 2009, 11, 3028.
doi: 10.1021/ol901041n |
| [13] |
(a) Foster, E. J.; Jones, R. B.; Lavigueur, C.; Williams, V. E. J. Am. Chem. Soc. 2006, 128, 8569.
pmid: 16802823 |
|
(b) Boden, N.; Bushby, R. J.; Lu, Z. B.; Cammidge, A. N. Liq. Cryst. 1999, 26, 495.
doi: 10.1080/026782999204930 pmid: 16802823 |
|
| [14] |
Feng, C.; Tian, X. L.; Zhou, J.; Xiang, S. K.; Yu, W. H.; Wang, B. Q.; Hu, P.; Redshaw, C.; Zhao, K. Q. Org. Biomol. Chem. 2014, 12, 6977.
doi: 10.1039/C4OB01503G |
| [15] |
(a) Han, X. D.; Tian, X. L.; Yu, W. H.; Xiang, S. K.; Hu, P.; Wang, B. Q.; Zhao, K. Q.; Chen, X. Z.; Feng, C. Liq. Cryst. 2017, 44, 1727.
doi: 10.1080/02678292.2017.1329557 |
|
(b) Feng, C.; Ding, Y. H.; Han, X. D.; Yu, W. H.; Xiang, S. K.; Wang, B. Q.; Hu, P.; Li, L. C.; Chen, X. Z.; Zhao, K. Q. Dyes Pigm. 2017, 139, 87.
doi: 10.1016/j.dyepig.2016.12.001 |
|
|
(c) Fan, S. Y.; Xu, H. T.; Li, Q. G.; Fang, D. M.; Yu, W. H.; Xiang, S. K.; Hu, P.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Liq. Cryst. 2019, 47, 1041.
doi: 10.1080/02678292.2019.1704898 |
|
|
(d) Du, Y.; Zhao, C. L.; Fan, S. Y.; Yu, W. H.; Xiang, S. K.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Liq. Cryst. 2022, 49, 719.
doi: 10.1080/02678292.2021.2006811 |
|
|
(e) Xia, X.; Zhao, C. L.; Xu, H. T.; Yu, W. H.; Xiang, S. K.; Li, L. C.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Dyes Pigm. 2022, 199, 110114.
doi: 10.1016/j.dyepig.2022.110114 |
|
| [16] |
Berg, R.; Straub, B. F. Beilstein J. Org. Chem. 2013, 9, 2715.
doi: 10.3762/bjoc.9.308 |
| [17] |
(a) Rodrigues, L. D.; Sunil, D.; Chaithra, D.; Bhagavath, P. J. Mol. Liq. 2020, 297, 111909.
doi: 10.1016/j.molliq.2019.111909 |
|
(b) Liu, Q. S.; Lü, Y. F.; Bao, P. L.; Yue, H. L.; Wei, W. Chin. J. Org. Chem. 2020, 40, 4015. (in Chinese)
doi: 10.6023/cjoc202008042 |
|
|
(刘启顺, 吕玉芬, 鲍鹏丽, 岳会兰, 魏伟, 有机化学, 2020, 40, 4015.)
doi: 10.6023/cjoc202008042 |
|
|
(c) Zhang, H. L.; Cheng, J. J.; Zou, G. J. Funct. Polym. 2019, 32, 728. (in Chinese)
|
|
|
(张红莉, 程军杰, 邹纲, 功能高分子学报, 2019, 32, 728.)
|
|
|
(d) Cheng, H. F. Ph.D. Dissertation, Yunnan University, Kunming, 2018. (in Chinese)
|
|
|
((程慧芳, 博士论文, 云南大学, 昆明, 2018.)
|
|
| [18] |
Gallardo, H.; Westphal, E. Curr. Org. Synth. 2015, 12, 806.
doi: 10.2174/157017941206150828113416 |
| [19] |
(a) Cheng, H. M.; Ma, C.; Chen, Y.; Ni, H. L.; Feng, C.; Wang, B. Q.; Zhao, K. Q.; Yu, W. H.; Hu, P. Liq. Cryst. 2017, 44, 1450.
doi: 10.1080/02678292.2017.1282047 |
|
(b) Xia, M. H.; Chen, Y.; Chen, Z. R.; Yu, W. H.; Cheng, H. M.; Feng, C.; Ni, H. L.; Wang, B. Q.; Zhao, K. Q.; Hu, P. Liq. Cryst. 2022, 49, 72.
doi: 10.1080/02678292.2021.1944358 |
|
|
(c) Xiong, D.; Xiong, X. P.; Chen, Z. R.; Yu, W. H.; Feng, C.; Chen, H. M.; Ni, H. L.; Wang, B. Q.; Zhao, K. Q.; Hu, P. Liq. Cryst. 2022, 49, 1261.
doi: 10.1080/02678292.2022.2066731 |
|
| [20] |
Benbayer, C.; Kheddam, N.; Saïdi-Besbes, S.; de Givenchy, E. T.; Guittard, F.; Grelet, E.; Safer, A. M.; Derdour, A. J. Mol. Struct. 2013, 1034, 22.
doi: 10.1016/j.molstruc.2012.09.020 |
| [21] |
Park, S.; Cho, B. K. Soft Matter 2015, 11, 94.
doi: 10.1039/C4SM02004A |
| [22] |
Bhalla, V.; Singh, H.; Kumar, M.; Prasad, S. K. Langmuir 2011, 27, 15275.
doi: 10.1021/la203774p |
| [23] |
(a) Yu, W. H.; Nie, S. C.; Bai, Y. F.; Jing, Y.; Wang, B. Q.; Hu, P.; Zhao, K. Q. Sci. China: Chem. 2010, 53, 1134.
doi: 10.1007/s11430-010-4010-3 |
|
(b) Neier, R.; Thevenet, D. Synthesis 2011, 2011, 3801.
doi: 10.1055/s-0031-1289573 |
|
| [24] |
Zhao, K. Q.; Bai, Y. F.; Hu, P.; Wang, B. Q.; Shimizu, Y. Mol. Cryst. Liq. Cryst. 2009, 509, 819.
|
| [25] |
Godbert, N.; Crispini, A.; Ghedini, M.; Carini, M.; Chiaravalloti, F.; Ferrise, A. J. Appl. Cryst. 2014, 47, 668.
doi: 10.1107/S1600576714003240 |
| [26] |
Yang, Y.; Wang, H.; Wang, H. F.; Liu, C. X.; Zhao, K. Q.; Wang, B. Q.; Hu, P.; Monobe, H.; Heinrich, B.; Donnio, B. Cryst. Growth Des. 2018, 18, 4296.
doi: 10.1021/acs.cgd.8b00083 |
| [1] | 胡立伟, 刘宪虎, 刘春太, 宋延林, 李明珠. 光子晶体结构色材料的自组装制备与应用★[J]. 化学学报, 2023, 81(7): 809-819. |
| [2] | 李兰英, 陶晴, 闻艳丽, 王乐乐, 郭瑞妍, 刘刚, 左小磊. 多聚腺嘌呤DNA探针及其生物传感应用★[J]. 化学学报, 2023, 81(6): 681-690. |
| [3] | 曾崇洋, 胡平, 汪必琴, 方文彦, 赵可清, Donnio Bertrand. 星型苯并菲-三嗪多刺激响应盘状液晶: 合成、性质与应用[J]. 化学学报, 2023, 81(5): 469-479. |
| [4] | 苏东芮, 任小康, 于沄淏, 赵鲁阳, 王天宇, 闫学海. 酪氨酸衍生物调控酶催化路径可控合成功能黑色素★[J]. 化学学报, 2023, 81(11): 1486-1492. |
| [5] | 溥旭, 李泽娟, 石隽秋, 朱云卿, 杜建忠. 器官靶向的聚合物核酸载体研究进展★[J]. 化学学报, 2023, 81(10): 1438-1446. |
| [6] | Jamshid Kadirkhanov, 钟峰, 张文建, 洪春雁. 聚合诱导自组装制备多腔室囊泡以及成核链段中亲溶剂片段的影响[J]. 化学学报, 2022, 80(7): 913-920. |
| [7] | 王志琴, 项博, 黄晓宇, 陆国林, 冯纯. 磷钨酸对对苯撑乙烯撑寡聚物-b-聚(2-乙烯基吡啶)自晶种行为的影响※[J]. 化学学报, 2022, 80(3): 297-302. |
| [8] | 郭湾, 胡聪意, 甄淑君, 黄承志, 李原芳. 铁基金属有机凝胶衍生的三氧化二铁纳米片用于光芬顿降解罗丹明B[J]. 化学学报, 2022, 80(12): 1583-1591. |
| [9] | 金艳梅, 蒙叶, 李远, 史建华, 邓雷. 对称二环己基取代六元瓜环与3-吡啶甲酰肼的超分子自组装[J]. 化学学报, 2022, 80(1): 44-48. |
| [10] | 王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣. 石墨烯量子点/铁基金属-有机骨架复合材料高效光催化二氧化碳还原※[J]. 化学学报, 2022, 80(1): 22-28. |
| [11] | 李卫华. 桥连对嵌段共聚物自组装的调控[J]. 化学学报, 2021, 79(2): 133-138. |
| [12] | 朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清. 苯并䓛盘状液晶: 合成、柱状相和光物理性质[J]. 化学学报, 2021, 79(12): 1486-1493. |
| [13] | 赵晶晶, 张正中, 陈小浪, 王蓓, 邓近远, 张蝶青, 李和兴. 微波诱导组装CuS@MoS2核壳纳米管及其光催化类芬顿反应研究[J]. 化学学报, 2020, 78(9): 961-967. |
| [14] | 江金辉, 朱云卿, 杜建忠. 开环聚合诱导自组装的挑战与展望[J]. 化学学报, 2020, 78(8): 719-724. |
| [15] | 尹岑, 王子宽, 刘丹, 彭展涛, 宋环君, 祝浩, 陈其伟, 吴凯. meso-四(对甲氧基苯基)卟啉钴在货币金属单晶表面的吸附与自组装研究[J]. 化学学报, 2020, 78(7): 695-702. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||