化学学报 ›› 2023, Vol. 81 ›› Issue (10): 1265-1270.DOI: 10.6023/A23050226 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
何倩†, 李杰†, 喻思佳, 吴东坪, 叶剑良*( ), 黄培强*(
), 黄培强*( )
)
投稿日期:2023-05-15
发布日期:2023-07-20
作者简介:基金资助:
Qian He†, Jie Li†, Sijia Yu, Dongping Wu, Jianliang Ye( ), Peiqiang Huang(
), Peiqiang Huang( )
)
Received:2023-05-15
Published:2023-07-20
Contact:
*E-mails: About author:Supported by:文章分享
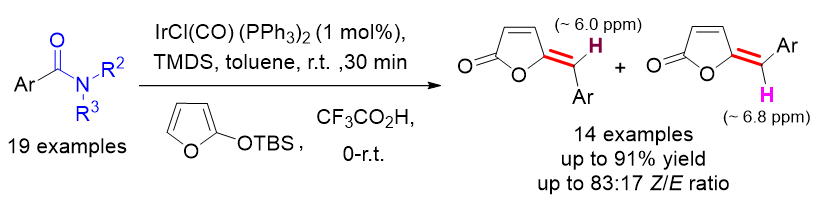
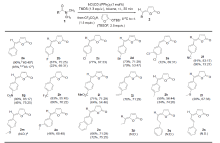
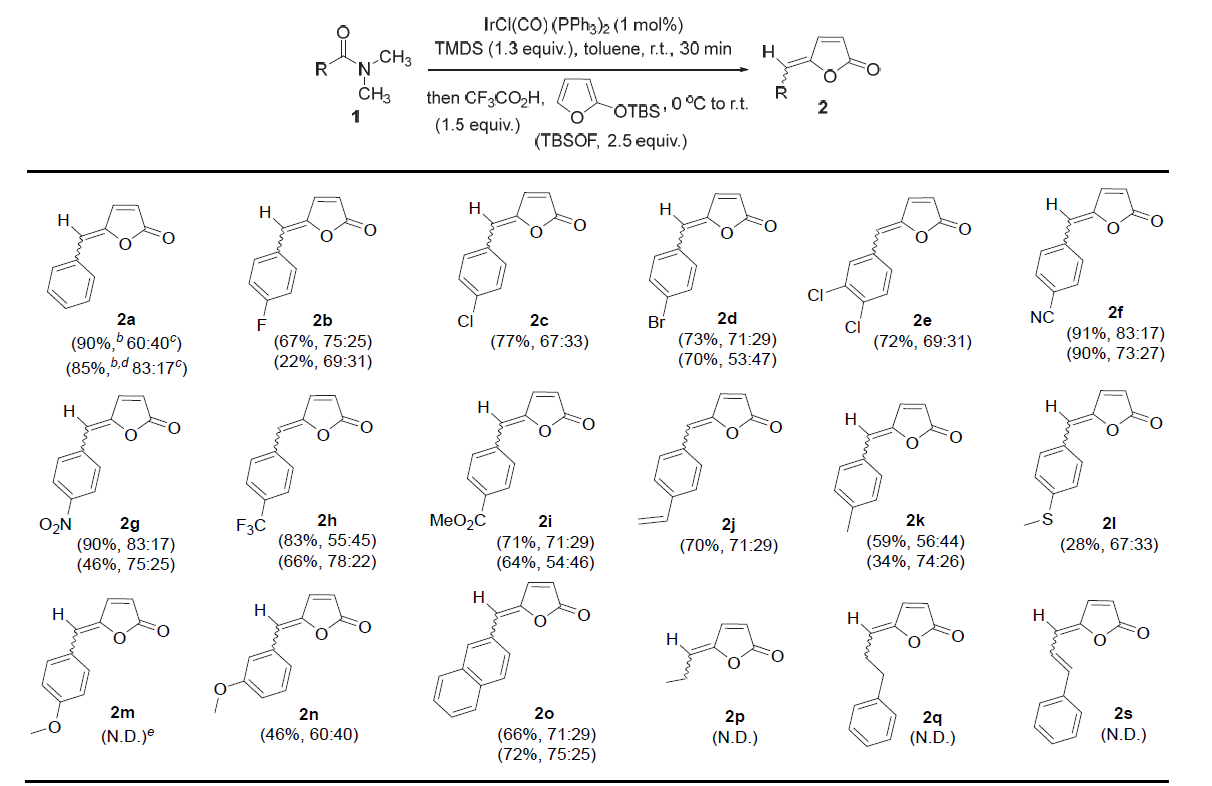
γ-亚烷基-丁烯酸内酯是许多具有重要生理活性天然产物的结构单元. 本工作通过铱催化N,N-二甲基芳基甲酰胺与呋喃硅醚间的类插烯Aldol缩合反应, 成功地实现了温和条件下γ-亚苄基-丁烯酸内酯的合成. 反应对硝基、氰基、乙烯基、三氟甲基等吸电子基取代的苯甲酰胺底物有很好的适用性. γ-亚苄基-丁烯酸内酯经Pd/C氢化反应即可转化为作为众多活性天然产物的核心骨架的γ-苄基丁内酯. 本工作还对Z型和E型γ-亚苄基-丁烯酸内酯的特征1H NMR谱峰的化学位移分布规律进行总结比较, 利用这些规律可以很方便地区分Z、E两种异构体.
何倩, 李杰, 喻思佳, 吴东坪, 叶剑良, 黄培强. 铱催化叔酰胺与呋喃硅醚间的类插烯Aldol缩合反应: γ-亚苄基-丁烯酸内酯的合成★[J]. 化学学报, 2023, 81(10): 1265-1270.
Qian He, Jie Li, Sijia Yu, Dongping Wu, Jianliang Ye, Peiqiang Huang. Ir-catalyzed Vinylogous Aldol-type Condensation Reactions between Tertiary Amides and Siloxyfuran: the Synthesis of γ-Benzylidenebutenolides★[J]. Acta Chimica Sinica, 2023, 81(10): 1265-1270.
| Entry | Acid/equiv. | TBSOF/equiv. | Timeb/h | Yield c/% | Z/Ed |
|---|---|---|---|---|---|
| 1 | BF3•Et2O (1.5) | 1.2 | 8 | 43 | 87∶13 |
| 2 | BF3•Et2O (1.5) | 2.0 | 8 | 63 | 88∶12 |
| 3 | BF3•Et2O (1.5) | 2.5 | 8 | 85 | 83∶17 |
| 4 | BF3•Et2O (1.5) | 3.5 | 8 | 65 | 86∶14 |
| 5 | TMSOTf (1.5) | 2.5 | 72 | N.D.e | — |
| 6 | Sc(OTf)3 (1.5) | 2.5 | 72 | N.D. | — |
| 7 | Cu(OTf)2 (1.5) | 2.5 | 72 | N.D. | — |
| 8 | ZnCl2 (1.5) | 2.5 | 10 | 26 | 75∶25 |
| 9 | AcOH (1.5) | 2.5 | 18 | 56 | 67∶33 |
| 10 | CF3CO2H (1.5) | 2.5 | 10 | 90 | 60∶40 |
| 11 | TfOH (1.5) | 2.5 | 10 | N.D. | — |
| 12 | CF3CO2H (1.0) | 2.5 | 10 | 69 | 59∶41 |
| 13 | CF3CO2H (2.0) | 2.5 | 10 | 47 | 60∶40 |
| Entry | Acid/equiv. | TBSOF/equiv. | Timeb/h | Yield c/% | Z/Ed |
|---|---|---|---|---|---|
| 1 | BF3•Et2O (1.5) | 1.2 | 8 | 43 | 87∶13 |
| 2 | BF3•Et2O (1.5) | 2.0 | 8 | 63 | 88∶12 |
| 3 | BF3•Et2O (1.5) | 2.5 | 8 | 85 | 83∶17 |
| 4 | BF3•Et2O (1.5) | 3.5 | 8 | 65 | 86∶14 |
| 5 | TMSOTf (1.5) | 2.5 | 72 | N.D.e | — |
| 6 | Sc(OTf)3 (1.5) | 2.5 | 72 | N.D. | — |
| 7 | Cu(OTf)2 (1.5) | 2.5 | 72 | N.D. | — |
| 8 | ZnCl2 (1.5) | 2.5 | 10 | 26 | 75∶25 |
| 9 | AcOH (1.5) | 2.5 | 18 | 56 | 67∶33 |
| 10 | CF3CO2H (1.5) | 2.5 | 10 | 90 | 60∶40 |
| 11 | TfOH (1.5) | 2.5 | 10 | N.D. | — |
| 12 | CF3CO2H (1.0) | 2.5 | 10 | 69 | 59∶41 |
| 13 | CF3CO2H (2.0) | 2.5 | 10 | 47 | 60∶40 |
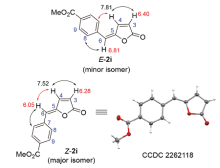
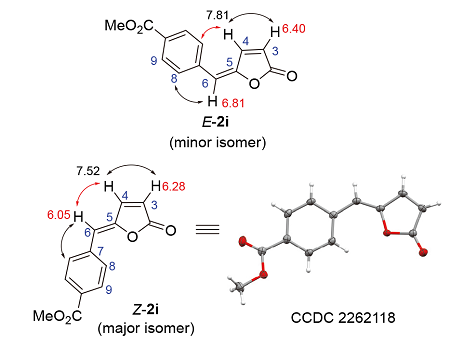
| Product | R | H-3 (δ) (d or dd peak) | H-6 (δ) (s peak) | H-3 (δ) (d or dd peak) | H-6 (δ) (s peak) |
|---|---|---|---|---|---|
| 2a | H | 6.34 | 6.80 | 6.17 | 6.01 |
| 2b | 4-F | 6.88 | 6.75 | 6.25 | 5.99 |
| 2c | 4-Cl | 6.87 | 6.74 | 6.23 | 5.98 |
| 2d | 4-Br | 6.40 | 6.74 | 6.24 | 5.96 |
| 2e | 3,4-diCl | 6.41 | 6.68 | 6.27 | 5.92 |
| 2f | 4-CN | 6.44 | 6.77 | 6.32 | 6.02 |
| 2g | 4-NO2 | 6.48 | 6.82 | 6.35 | 6.08 |
| 2h | 4-CF3 | 6.42 | 6.81 | 6.28 | 6.05 |
| 2i | 4-CO2Me | 6.40 | 6.81 | 6.28 | 6.05 |
| 2j | 4-vinyl | 6.34 | 6.77 | 6.19 | 6.00 |
| 2k | 4-Me | 6.34 | 6.80 | 6.21 | 6.03 |
| 2l | 4-MeS | 6.35 | 6.76 | 6.21 | 6.00 |
| 2n | 3-MeO | 6.34 | 6.77 | 6.22 | 6.00 |
| 2oa | 6.33 | 7.38 | 6.26 | 6.81 |
| Product | R | H-3 (δ) (d or dd peak) | H-6 (δ) (s peak) | H-3 (δ) (d or dd peak) | H-6 (δ) (s peak) |
|---|---|---|---|---|---|
| 2a | H | 6.34 | 6.80 | 6.17 | 6.01 |
| 2b | 4-F | 6.88 | 6.75 | 6.25 | 5.99 |
| 2c | 4-Cl | 6.87 | 6.74 | 6.23 | 5.98 |
| 2d | 4-Br | 6.40 | 6.74 | 6.24 | 5.96 |
| 2e | 3,4-diCl | 6.41 | 6.68 | 6.27 | 5.92 |
| 2f | 4-CN | 6.44 | 6.77 | 6.32 | 6.02 |
| 2g | 4-NO2 | 6.48 | 6.82 | 6.35 | 6.08 |
| 2h | 4-CF3 | 6.42 | 6.81 | 6.28 | 6.05 |
| 2i | 4-CO2Me | 6.40 | 6.81 | 6.28 | 6.05 |
| 2j | 4-vinyl | 6.34 | 6.77 | 6.19 | 6.00 |
| 2k | 4-Me | 6.34 | 6.80 | 6.21 | 6.03 |
| 2l | 4-MeS | 6.35 | 6.76 | 6.21 | 6.00 |
| 2n | 3-MeO | 6.34 | 6.77 | 6.22 | 6.00 |
| 2oa | 6.33 | 7.38 | 6.26 | 6.81 |
| [1] |
(a) Pace, V.; Holzer, W. Aust. J. Chem. 2013, 66, 507.
doi: 10.1071/CH13042 |
|
(b) Pace, V.; Holzer, W.; Olofsson, B. Adv. Synth. Catal. 2014, 356, 3697.
doi: 10.1002/adsc.v356.18 |
|
|
(c) Volkov, A.; Tinnis, F.; Slagbrand, T.; Trillo, P.; Adolfsson, H. Chem. Soc. Rev. 2016, 45, 6685.
doi: 10.1039/C6CS00244G |
|
|
(d) Sato, T.; Yoritate, M.; Tajima, H.; Chida, N. Org. Biomol. Chem. 2018, 16, 3864.
doi: 10.1039/C8OB00733K |
|
|
(e) Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Chem. Soc. Rev. 2018, 47, 7899.
doi: 10.1039/C8CS00335A |
|
|
(f) Matheau-Raven, D.; Gabriel, P.; Leitch, J. A.; Almehmadi, Y. A.; Yamazaki, K.; Dixon, D. J. ACS Catal. 2020, 8880.
|
|
| [2] |
(a) Xiao, K.-J.; Luo, J.-M.; Ye, K.-Y.; Wang, Y.; Huang, P.-Q. Angew. Chem., Int. Ed. 2010, 49, 3037.
doi: 10.1002/anie.v49:17 |
|
(b) Gregory, A. W.; Chambers, A.; Hawkins, A.; Jakubec, P.; Dixon, D. J. Chem. - Eur. J. 2015, 21, 111.
doi: 10.1002/chem.v21.1 |
|
|
(c) Huang, P.-Q.; Ou, W.; Han, F. Chem. Commun. 2016, 52, 11967.
doi: 10.1039/C6CC05318A |
|
|
(d) Tinnis, F.; Volkov, A.; Slagbrand, T.; Adolfsson, H. Angew. Chem., Int. Ed. 2016, 55, 4562.
doi: 10.1002/anie.v55.14 |
|
|
(e) Xie, L.-G.; Dixon, D. J. Chem. Sci. 2017, 8, 7492.
doi: 10.1039/C7SC03613B |
|
|
(f) Takahashi, Y.; Sato, T.; Chida, N. Chem. Lett. 2019, 48, 1138.
doi: 10.1246/cl.190467 |
|
|
(g) Ou, W.; Huang, P.-Q. Sci. China: Chem. 2020, 63, 11.
|
|
|
(h) Yamazaki, K.; Gabriel, P.; Di Carmine, G.; Pedroni, J.; Farizyan, M.; Hamlin, T. A.; Dixon, D. J. ACS Catal. 2021, 11, 7489.
doi: 10.1021/acscatal.1c01589 |
|
|
(i) Jiao, J.-W.; Wang, X.-M. Angew. Chem., Int. Ed. 2021, 60, 17088.
doi: 10.1002/anie.v60.31 |
|
|
(j) Biallas, P.; Yamazaki, K.; Dixon, D. J. Org. Lett. 2022, 24, 2002.
doi: 10.1021/acs.orglett.2c00438 |
|
|
(k) Wu, D.-P.; Ou, W.; Huang, P.-Q. Org. Lett. 2022, 24, 5366.
doi: 10.1021/acs.orglett.2c02045 |
|
|
(l) Zhao, F.; Jiang, F.; Wang, X.-M. Sci. China: Chem. 2022, 65, 2231.
|
|
|
(m) He, Y.-L.; Wang, Y.-X.; Li, S.-J.; Lan, Y.; Wang, X.-M. Angew. Chem., Int. Ed. 2022, 61, e202115497.
|
|
|
(n) Yang, W.-H.; Jiao, J.-W.; Wang, X.-M. Chin. J. Org. Chem. 2023, 43, 1857 (in Chinese).
doi: 10.6023/cjoc202212019 |
|
|
(杨雯涵, 焦继文, 王晓明, 有机化学, 2023, 43, 1857.)
|
|
| [3] |
(a) Soda, Y.; Sugiyama, Y.; Sato, S.; Shibuya, K.; Saegusa, J.; Matagawa, T.; Kawano, S.; Yoritate, M.; Fukaya, K.; Urabe, D.; Oishi, T.; Mori, K.; Simizu, S.; Chida, N.; Sato, T. Synthesis 2023, 55, 617.
doi: 10.1055/a-1941-8680 |
|
(b) Soda, Y.; Sugiyama, Y.; Yoritate, M.; Tajima, H.; Shibuya, K.; Ogihara, C.; Oishi, T.; Sato, T.; Chida, N. Org. Lett. 2020, 22, 7502.
doi: 10.1021/acs.orglett.0c02697 |
|
|
(c) Huang, X.-Z.; Gao, L.-H.; Huang, P.-Q. Nat. Commun. 2020, 11, 5314.
doi: 10.1038/s41467-020-19163-4 |
|
|
(d) Yoritate, M.; Takahashi, Y.; Tajima, H.; Ogihara, C.; Yokoyama, T.; Soda, Y.; Oishi, T.; Sato, T.; Chida, N. J. Am. Chem. Soc. 2017, 139, 18386.
doi: 10.1021/jacs.7b10944 |
|
|
(e) Guo, L.-D.; Hou, J.; Tu, W.; Zhang, Y.; Zhang, Y.; Chen, L.; Xu, J. J. Am. Chem. Soc. 2019, 141, 11713.
doi: 10.1021/jacs.9b05641 |
|
|
(f) Guo, L.-D.; Chen, Y.; Xu, J. Acc. Chem. Res. 2020, 53, 2726.
doi: 10.1021/acs.accounts.0c00532 |
|
|
(g) Huang, Y.-H.; Liu, Z.-J.; Huang, P.-Q. Org. Chem. Front. 2022, 9, 58.
doi: 10.1039/D1QO01408K |
|
|
(h) Hu, J.-P.; Chen, W.-Q.; Jiang, Y.-Y.; Xu, J. Chin. J. Org. Chem. 2023, 43, 171 (in Chinese).
doi: 10.6023/cjoc202208014 |
|
|
(胡晶平, 陈文清, 蒋宇旸, 徐晶, 有机化学, 2023, 43, 171.)
|
|
| [4] |
(a) Ruan, S.-T.; Luo, J.-M.; Du, Y.; Huang, P.-Q. Org. Lett. 2011, 13, 4938.
doi: 10.1021/ol2020384 |
|
(b) Ye, J.-L.; Zhang, Y.-F.; Liu, Y.; Zhang, J.-Y.; Ruan, Y.-P.; Huang, P.-Q. Org. Chem. Front. 2015, 2, 697.
doi: 10.1039/C5QO00098J |
|
| [5] |
(a) Chen, H.; Wu, Z.-Z.; Shao, D.-Y.; Huang, P.-Q. Sci. Adv. 2022, 8, eade3431.
|
|
(b) Ou, W.; Han, F.; Hu, X.-N.; Chen, H.; Huang, P.-Q. Angew. Chem., Int. Ed. 2018, 57, 11354.
doi: 10.1002/anie.v57.35 |
|
|
(c) Ji, K.-L.; He, S.-F.; Xu, D.-D.; He, W.-X.; Zheng, J.-F.; Huang, P.-Q. Angew. Chem., Int. Ed., 2023, 63, e202302832.
|
|
| [6] |
Nakajima, M.; Sato, T.; Chida, N. Org. Lett. 2015, 17, 1696.
doi: 10.1021/acs.orglett.5b00664 |
| [7] |
(a) Chatterjee, S.; Sahoo, R.; Nanda, S. Org. Biomol. Chem. 2021, 19, 7298.
doi: 10.1039/D1OB00875G |
|
(b) Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. J. Antibiot. 2014, 67, 315.
doi: 10.1038/ja.2013.135 |
|
|
(c) Smitha, D.; Kumar, M. M. K.; Ramana, H.; Rao, D. V. Nat. Prod. Res. 2014, 28, 12.
doi: 10.1080/14786419.2013.827194 |
|
|
(d) Sikorska, J.; Parker-Nance, S.; Davies-Coleman, M. T.; Vining, O. B.; Sikora, A. E.; McPhail, K. L. J. Nat. Prod. 2012, 75, 1824.
doi: 10.1021/np300580z |
|
|
(e) Wang, W.; Kim, H.; Nam, S.-J.; Rho, B. J.; Kang, H. J. Nat. Prod. 2012, 75, 2049.
doi: 10.1021/np300544a |
|
|
(f) Teixeira, R. R.; Barbosa, L. C. A.; Maltha, C. R. A.; Rocha, M. E.; Bezerra, D. P.; Costa-Lotufo, L. V.; Pessoa, C.; Moraes, M. O. Molecules 2007, 12, 1101.
doi: 10.3390/12051101 |
|
|
(g) Manzanaro, S.; Salvá, J.; de la Fuente, J. Á. J. Nat. Prod. 2006, 69, 1485.
doi: 10.1021/np0503698 |
|
|
(h) Fang, X. P.; Anderson, J. E.; Chang, C. J.; McLaughlin, J. L. Tetrahedron 1991, 47, 9751.
doi: 10.1016/S0040-4020(01)80715-8 |
|
|
(i) Miao, S.; Andersen, R. J. J. Org. Chem. 1991, 56, 6275.
doi: 10.1021/jo00022a012 |
|
|
(j) Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. 1984, 23, 445.
doi: 10.1002/anie.v23:6 |
|
| [8] |
Aumann, D. C.; Clooth, G.; Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. 1989, 28, 453.
doi: 10.1002/anie.v28:4 |
| [9] |
(a) Guo, Z.; Bao, R.; Li, Y.; Li, Y.; Zhang, J.; Tang, Y. Angew. Chem., Int. Ed. 2021, 60, 14545.
doi: 10.1002/anie.v60.26 |
|
(b) Yang, M.; Yin, F.; Fujino, H.; Snyder, S. A. J. Am. Chem. Soc. 2019, 141, 4515.
doi: 10.1021/jacs.8b12612 |
|
| [10] |
(a) Roy, D.; Tharra, P.; Baire, B. Org. Lett. 2021, 23, 5605.
doi: 10.1021/acs.orglett.1c01529 |
|
(b) Li, B.-Z.; Chen, W.-M. Chin. J. Org. Chem. 2008, 28, 29 (in Chinese).
|
|
|
(李冰洲, 陈卫民, 有机化学, 2008, 28, 29.)
|
|
|
(c) Wang, B.-W.; Liu, Y.; Hao, Z.-F.; Hou, J.-Q.; Li, J.-Y.; Li, S.-T.; Pan, S.-H.; Zeng, M.-H.; Wang, Z.-Y. Chin. J. Org. Chem. 2018, 38, 1872 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
(王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳, 有机化学, 2018, 38, 1872.)
|
|
| [11] |
Coperet, C.; Sugihara, T.; Wu, G.; Shimoyama, I.; Negishi, E.-I. J. Am. Chem. Soc. 1995, 117, 3422.
doi: 10.1021/ja00117a011 |
| [12] |
Inack-Ngi, S.; Rahmani, R.; Commeiras, L.; Chouraqui, G.; Thibonnet, J.; Duchêne, A.; Abarbri, M.; Parrain, J.-L. Adv. Synth. Catal. 2009, 351, 779.
doi: 10.1002/adsc.v351:5 |
| [13] |
(a) Chatterjee, S.; Acharyya, R. K.; Pal, P.; Nanda, S. Org. Biomol. Chem. 2022, 20, 2473.
doi: 10.1039/D2OB00166G |
|
(b) Rambabu, D.; Bhavani, S.; Nalivela, K. S.; Mukherjee, S.; Rao, M. V. B.; Pal, M. Tetrahedron Lett. 2013, 54, 2151.
doi: 10.1016/j.tetlet.2013.02.040 |
|
|
(c) Lu, X.; Huang, X.; Ma, S. Tetrahedron Lett. 1993, 34, 5963.
doi: 10.1016/S0040-4039(00)73827-5 |
|
| [14] |
Yu, C.; Zhang, J.; Zhong, G. Chem. Commun. 2017, 53, 9902.
doi: 10.1039/C7CC04973K |
| [15] |
(a) Casiraghi, G.; Zanardi, F.; Appendino, G.; Rassu, G. Chem. Rev. 2000, 100, 1929.
doi: 10.1021/cr990247i |
|
(b) Khotavivattana, T.; Khamkhenshorngphanuch, T.; Rassamee, K.; Siripong, P.; Vilaivan, T. Tetrahedron Lett. 2018, 59, 2711.
doi: 10.1016/j.tetlet.2018.06.005 |
|
|
(c) Kuse, M.; Moriguchi, M.; Hachida, M.; Takikawa, H. Chem. Lett. 2017, 46, 1409.
doi: 10.1246/cl.170631 |
|
|
(d) Chaubet, G.; Goh, S. S.; Mohammad, M.; Gockel, B.; Cordonnier, M.-C. A.; Baars, H.; Phillips, A. W.; Anderson, E. A. Chem. - Eur. J. 2017, 23, 14080.
doi: 10.1002/chem.v23.56 |
|
|
(e) Boukouvalas, J.; Beltran, P. P.; Lachance, N.; Cote, S.; Maltais, F.; Pouliot, M. Synlett 2007, 219.
|
|
|
(f) Kong, K.; Romo, D. Org. Lett. 2006, 8, 2909.
doi: 10.1021/ol060534q |
|
|
(g) Vaz, B.; Alvarez, R.; Brückner, R.; de Lera, A. R. Org. Lett. 2005, 7, 545.
doi: 10.1021/ol0478281 |
|
|
(h) Fiorenza, M.; Reginato, G.; Ricci, A.; Taddei, M.; Dembech, P. J. Org. Chem. 1984, 49, 551.
doi: 10.1021/jo00177a034 |
|
|
(i) Asaoka, M.; Yanagida, N.; Ishibashi, K.; Takei, H. Tetrahedron Lett. 1981, 22, 4269.
doi: 10.1016/S0040-4039(01)82929-4 |
|
| [16] |
(a) Schacht, M.; Boehlich, G. J.; de Vries, J.; Bertram, S.; Gabriel, G.; Zimmermann, P.; Heisig, P.; Schuetzenmeister, N. Eur. J. Org. Chem. 2017, 1745.
|
|
(b) Teixeira, R. R.; Barbosa, L. C. A.; Forlani, G.; Pilo-Veloso, D.; Carneiro, J. W. M. J. Agric. Food Chem. 2008, 56, 2321.
doi: 10.1021/jf072964g |
|
|
(c) Teixeira, R. R.; Barbosa, L. C. A.; Santana, J. O.; Veloso, D. P.; Ellena, J.; Doriguetto, A. C.; Drew, M. G. B.; Ismail, F. M. D. J. Mol. Struct. 2007, 837, 197.
doi: 10.1016/j.molstruc.2006.10.014 |
|
| [17] |
Kotora, M.; Negishi, E. I. Synthesis 1997, 121.
|
| [18] |
Lambert, J. D.; Rice, J. E.; Hong, J.; Hou, Z.; Yang, C. S. Bioorg. Med. Chem. Lett. 2005, 15, 873.
doi: 10.1016/j.bmcl.2004.12.070 |
| [19] |
(a) Gogoi, S.; Argade, N. P. Tetrahedron 2006, 62, 2715.
doi: 10.1016/j.tet.2005.12.020 |
|
(b) Wu, L.; Zhang, Z.; Liao, J.; Li, J.; Wu, W.; Jiang, H. Chem. Commun. 2016, 52, 2628.
doi: 10.1039/C5CC08867D |
|
| [20] |
(a) Li, M.-L.; Li, Y.; Pan, J.-B.; Li, Y.-H.; Song, S.; Zhu, S.-F.; Zhou, Q.-L. ACS Catal. 2020, 10, 10032.
doi: 10.1021/acscatal.0c02142 |
|
(b) Shu, C.; Liu, M.-Q.; Sun, Y.-Z.; Ye, L.-W. Org. Lett. 2012, 14, 4958.
doi: 10.1021/ol302323a |
| [1] | 赵庆如, 蒋茹, 游书力. 铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物[J]. 化学学报, 2021, 79(9): 1107-1112. |
| [2] | 杨普苏, 刘晨旭, 张文文, 游书力. 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021, 79(6): 742-746. |
| [3] | 杨妲, 张龙力, 刘欢, 杨朝合. 双功能配体修饰的Ir催化剂在“氢甲酰化-缩醛化”串联反应中的共催化作用[J]. 化学学报, 2021, 79(5): 658-662. |
| [4] | 翁维正, 罗春容, 李建梅, 刘颖, 林海强, 黄传敬, 万惠霖. Ir/SiO2上甲烷部分氧化制合成气反应的原位时间分辨红外和原位Raman光谱表征[J]. 化学学报, 2004, 62(18): 1853-1857. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||