化学学报 ›› 2023, Vol. 81 ›› Issue (12): 1716-1723.DOI: 10.6023/A23070349 上一篇 下一篇
研究论文
贾彦荣a, 徐凯a, 赵彦英a, 倪华钢a, 吴滢b, 夏敏a,*( )
)
投稿日期:2023-07-20
发布日期:2023-10-31
基金资助:
Yanrong Jiaa, Kai Xua, Yanying Zhaoa, Huagang Nia, Ying Wub, Min Xiaa( )
)
Received:2023-07-20
Published:2023-10-31
Contact:
*E-mail: Supported by:文章分享

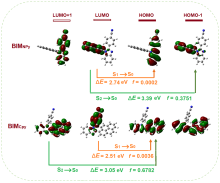
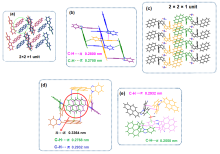
以芘基和4-腈基苯基为取代基, 分别合成了两者在苯并咪唑环上N1位与C2位区域异构的两个化合物BIMNPy和BIMCPy, 研究了两种化合物在溶液中以及固态下的荧光发射性质与力致荧光变色行为. 在溶液中, BIMCPy的荧光发射波长表现出中等幅度的正向溶剂变色效应, 而BIMNPy的荧光发射波长则对溶剂极性的变化无响应. 两种化合物的晶体在受力后均出现发射波长的蓝移, 且在BIMNPy晶体上产生的力致荧光变色活性比在BIMCPy晶体上更为显著. 此外, BIMNPy的力致荧光变色性在溶剂熏蒸及热退火状态下均可逆, 并在室温下具有部分自恢复特性; BIMCPy的力致荧光变色性在溶剂熏蒸下可逆而在热退火处理时不可逆. 根据示差热扫描、粉末与单晶衍射、发射衰变光谱以及理论计算的结果, 对两种化合物发射行为差异性的原因进行了分析与探讨, 认为溶液态下的差异起源于高能级电子激发态的发射特征不同而固态下的差异起源于分子堆积模式的区别, 但推拉电子基团区域异构所引起的分子偶极矩以及π-共轭程度的变化则是异构体产生宏观性能不同的根本原因.
贾彦荣, 徐凯, 赵彦英, 倪华钢, 吴滢, 夏敏. 推拉电子基团区域异构苯并咪唑的发射行为与力致荧光变色性能研究[J]. 化学学报, 2023, 81(12): 1716-1723.
Yanrong Jia, Kai Xu, Yanying Zhao, Huagang Ni, Ying Wu, Min Xia. Research on Emission Behavior and Mechanofluorochromic Activity of Benzo[d]imidazoles with Region-isomerized Push-Pull Groups[J]. Acta Chimica Sinica, 2023, 81(12): 1716-1723.









| [1] |
(a) Peng, B.-Y.; Xu, S.-D.; Chi, Z.-G.; Zhang, X.-Q.; Zhang, Y.; Xu, J.-R. Prog. Chem. 2013, 25, 1805 (in Chinese).
|
|
(彭邦银, 许适当, 池振国, 张锡奇, 张艺, 许家瑞, 化学进展, 2013, 25, 1805);
doi: 10.7536/PC130329 |
|
|
(b) Di, B. H.; Chen, Y. L. Chinese Chem. Lett. 2018, 29, 245;
doi: 10.1016/j.cclet.2017.08.043 |
|
|
(c) Yuan, Y,; Yuan, W.; Chen, Y. L. Sci. China Mater. 2016, 59, 507;
doi: 10.1007/s40843-016-5060-7 |
|
|
(d) Yuan, W.; Yuan, Y.; Chen, Y.-L. Acta Polym. Sinica 2016, 11, 1495 (in Chinese).
|
|
|
(袁伟, 袁媛, 陈于蓝, 高分子学报, 2016, 11, 1495);
|
|
|
(e) Yang, J.; Chi, Z.; Zhu, W.; Tang, B. Z.; Li, Z. Sci. China Chem. 2019, 62, 1090;
doi: 10.1007/s11426-019-9512-x |
|
|
(f) Li, Q.; Li, Z. Adv. Sci. 2017, 4, 1600484;
doi: 10.1002/advs.v4.7 |
|
|
(g) Tsuchiya, Y.; Yamaguchi, K.; Miwa, Y.; Kutsumizu, S.; Minoura, M.; Murai, T. Bull. Chem. Soc. Jpn. 2020, 93, 927;
doi: 10.1246/bcsj.20200083 |
|
|
(h) Barman, D.; Gogoi, R.; Narang, K.; Iyer, P. K. Front. Chem. 2020, 8, 483;
doi: 10.3389/fchem.2020.00483 |
|
|
(i) Wang, J.-F.; Li, Z. Acta Chim. Sinica 2021, 79, 575 (in Chinese).
doi: 10.6023/A21010029 |
|
|
(王金凤, 李振, , 化学学报, 2021, 79, 575);
doi: 10.6023/A21010029 |
|
|
(j) Wang, Z.; Liu, L.; Xu, B.; Tian, W. Chem. Res. Chinese Univ. 2021, 37, 100.
doi: 10.1007/s40242-021-0431-0 |
|
| [2] |
(a) Shi, P.; Duan, Y.; Wei, W.; Xu, Z.; Li, Z.; Han, T. J. Mater. Chem. C 2018, 6, 2476;
doi: 10.1039/C7TC05683D |
|
(b) Feng, C.; Wang, K.; Xu, Y.; Liu, L.; Zou, B.; Lu, P. Chem. Commun. 2016, 52, 3836;
doi: 10.1039/C5CC09152G |
|
|
(c) Wang, L.; Wang, K.; Zou, B.; Ye, K.; Zhang, H.; Wang, Y. Adv. Mater. 2015, 27, 2918;
doi: 10.1002/adma.201500589 |
|
|
(d) Xie, W.-Z.; Zheng, H.-C.; Zheng, Y.-S. J. Mater. Chem. C 2017, 5, 10462.
doi: 10.1039/C7TC02525D |
|
| [3] |
(a) Hirata, S.; Watanabe, T. Adv. Mater. 2006, 18, 2725;
doi: 10.1002/adma.v18:20 |
|
(b) Lim, S. J.; An, B. K.; Jung, S. D.; Chung, M. A.; Park, S. Y. Angew. Chem., Int. Ed. 2004, 43, 6346;
doi: 10.1002/anie.v43:46 |
|
|
(c) Olson, C. E.; Previte, M. J. R.; Fourkas, J. T. Nat. Mater. 2002, 1, 225;
doi: 10.1038/nmat766 |
|
|
(d) Irie, M.; Fukaminato, T.; Sasaki, T.; Tamai, N.; Kawai, T. Nature 2002, 420, 759.
doi: 10.1038/420759a |
|
| [4] |
(a) Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Nat. Mater. 2005, 4, 546;
pmid: 15965481 |
|
(b) Zhu, X.; Liu, R.; Li, Y.; Huang, H.; Wang, Q.; Wang, D.; Zhu, X.; Liu, S.; Zhu, H. Chem. Commun. 2014, 50, 12951;
doi: 10.1039/C4CC05913A pmid: 15965481 |
|
|
(c) Qi, Q.; Liu, Y.; Fang, X.; Zhang, Y.; Chen, P.; Wang, Y.; Yang, B.; Xu, B.; Tian, W.; Zhang, S. X. RSC Adv. 2013, 3, 7996;
doi: 10.1039/c3ra40734a pmid: 15965481 |
|
|
(d) Kumar, P.; Dwivedi, J.; Gupta, B. K. J. Mater. Chem. C, 2014, 2, 10468;
doi: 10.1039/C4TC02065K pmid: 15965481 |
|
|
(e) Lu, X.-L.; Xia, M. J. Mater. Chem. C, 2016, 4, 9350.
doi: 10.1039/C6TC02618D pmid: 15965481 |
|
| [5] |
(a) Yuan, W. Z.; Tan, Y.; Gong, Y.; Lu, P.; Lam, J. W. Y.; Shen, X. Y.; Feng, C.; Sung, H. Y.; Lu, Y.; Williams, I. D.; Sun, J. Z.; Zhang, Y.; Tang, B. Z. Adv. Mater. 2013, 25, 2837;
doi: 10.1002/adma.v25.20 |
|
(b) Li, C.; Tang, X.; Zhang, L.; Li, C.; Liu, Z.; Bo, Z.; Dong, Y. Q.; Tian, Y.-H.; Dong, Y.; Tang, B. Z. Adv. Optical Mater. 2015, 3, 1184;
doi: 10.1002/adom.v3.9 |
|
|
(c) Naeem, K. C.; Subhakumari, A.; Varughese, S.; Nair, V. C. J. Mater. Chem. C 2015, 3, 10225;
doi: 10.1039/C5TC02062J |
|
|
(d) Sun, J.; Han, J.; Liu, Y.; Duan, Y.; Han, T.; Yuan, J. J. Mater. Chem. C 2016, 4, 8276;
doi: 10.1039/C6TC03428D |
|
|
(e) Xue, P.; Yang, Z.; Chen, P. J. Mater. Chem. C 2018, 6, 4994.
doi: 10.1039/C8TC01379A |
|
| [6] |
(a) Sagara, Y.; Kato, T. Angew. Chem. Int. Ed. 2011, 50, 9128;
doi: 10.1002/anie.v50.39 |
|
(b) Yoon, S.-J.; Chung, J. W.; Gierschner, J.; Kim, K. S.; Choi, M.-G.; Kim, D.; Park, S. Y. J. Am. Chem. Soc. 2010, 132, 13675;
doi: 10.1021/ja1044665 |
|
|
(c) Sun, H.; Liu, S.; Lin, W.; Zhang, K. Y.; Lv, W.; Huang, W.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; Huang, W. Nat. Commun. 2014, 5, 3601;
doi: 10.1038/ncomms4601 |
|
|
(d) Zhang, K. Y.; Liu, S.; Zhao, Q.; Huang, W. Coord. Chem. Rev. 2016, 319, 180;
doi: 10.1016/j.ccr.2016.03.016 |
|
|
(e) Chen, X.; Sun, G.; Zhang, T.; Liu, S.; Zhao, Q.; Huang, W. Adv. Mater. 2016, 28, 7137;
doi: 10.1002/adma.v28.33 |
|
|
(f) Zhao, Q.; Xu, W.; Sun, H.; Yang, J.; Zhang, K. Y.; Liu, S.; Ma, Y.; Huang, W. Adv. Opt. Mater. 2016, 4, 1167;
doi: 10.1002/adom.v4.8 |
|
|
(g) Lin, W.; Zhao, Q.; Sun, H.; Zhang, K. Y.; Yang, H.; Yu, Q.; Zhou, X.; Guo, S.; Liu, S.; Huang, W. Adv. Opt. Mater. 2015, 3, 368;
doi: 10.1002/adom.v3.3 |
|
|
(h) Han, J.; Sun, J.; Li, Y.; Duan, Y.; Han, T. J. Mater. Chem. C 2016, 4, 9287.
doi: 10.1039/C6TC03131E |
|
| [7] |
(a) Yang, W.; Yang, Y.; Qiu, Y.; Cao, X.; Huang, Z.; Gong, S.; Yang, C. Mater. Chem. Front. 2020, 4, 2047;
doi: 10.1039/D0QM00247J |
|
(b) Liu, X.-J.; Jiang, H.; Jia, Y.-R.; Xia, M. Dyes & Pigm. 2020, 172, 107845;
|
|
|
(c) Wu, J.; Cheng, Y.; Lan, J.; Wu, D.; Qian, S.; Yan, L.; He, Z.; Li, X.; Wang, K.; Zou, B.; You, J. J. Am. Chem. Soc. 2016, 138, 12803.
doi: 10.1021/jacs.6b03890 |
|
| [8] |
(a) Song, Q.; Wang, Y.; Hu, C.; Zhang, Y.; Sun, J.; Wang, K.; Zhang, C. New J. Chem. 2015, 39, 659;
doi: 10.1039/C4NJ01492H |
|
(b) Ekbote, A.; Mobin, S. M.; Misra, R. J. Mater. Chem. C 2020, 8, 3589;
doi: 10.1039/C9TC05192A |
|
|
(c) Li, W.; Wang, L.; Zhang, J. P. Wang, H. J. Mater. Chem. C 2014, 2, 1887;
doi: 10.1039/c3tc31974a |
|
|
(d) Jiao, Y.; Li, M.; Wang, N.; Lu, T.; Zhou, L.; Huang, Y.; Lu, Z.; Luo, D.; Pu, X. J. Mater. Chem. C 2016, 4, 4269.
doi: 10.1039/C6TC00153J |
|
| [9] |
(a) Liu, X.-J.; Jiang, H.; Jia, Y.-R.; Xia, M. RSC Adv. 2020, 10, 23187;
doi: 10.1039/D0RA02737E |
|
(b) Gao, G.-L.; Jia, Y.-R.; Jiang, H.; Xia, M. Dyes & Pigm. 2021, 186, 109030;
|
|
|
(c) Jia, Y.-R.; Jiang, H.; Gao, G.-L.; Xu, K.; Xia, M. Dyes & Pigm. 2021, 194, 109541;
|
|
|
(d) Liu, X.-J.; Jia, Y.-R.; Jiang, H.; Gao, G.-L.; Xia, M. Acta Chim. Sinica 2019, 77, 1194 (in Chinese).
doi: 10.6023/A19080306 |
|
|
(刘笑静, 贾彦荣, 江豪, 高贯雷, 夏敏, 化学学报, 2019, 77, 1194);
doi: 10.6023/A19080306 |
|
|
(e) Wang, Z.-Y.; Zhao, J.-W.; Li, P.; Feng, T.; Wang, W.-J.; Tao, S.-L.; Tong, Q.-X. New J. Chem. 2018, 42, 8924;
doi: 10.1039/C8NJ01006D |
|
|
(f) Jadhav, T.; Choi, J. M.; Dhokale, B.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Phys. Chem. C, 2016, 120, 18487;
doi: 10.1021/acs.jpcc.6b06277 |
|
|
(g) Jadhav, T.; Choi, J. M.; Shinde, J.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2017, 5, 6014;
doi: 10.1039/C7TC00950J |
|
|
(h) Ekbote, A.; Han, S. H.; Jadhav, T.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2018, 6, 2077;
doi: 10.1039/C7TC05450E |
|
|
(i) Zhan, Y.; Xu, Y.; Jin, Z.; Ye, W.; Yang, P. Dyes & Pigm. 2017, 140, 452;
|
|
|
(j) Gao, Z.; Wang, K.; Liu, F.; Feng, C.; He, X.; Li, J.; Yang, B.; Zou, B.; Lu, P. Chem. Eur. J. 2017, 23, 773;
doi: 10.1002/chem.v23.4 |
|
|
(k) Nagai, S.; Yamashita, M.; Tachikawa, T.; Ubukata, T.; Asami, M.; Ito, S. J. Mater. Chem. C 2019, 7, 4988;
doi: 10.1039/C9TC00157C |
|
|
(l) Lu, G.; Luo, N.; Hu, F.; Ban, Z.; Zhan, Z.; Huang, G.-S. Adv. Syn. Cat. 2020, 362, 487.
doi: 10.1002/adsc.v362.3 |
|
| [10] |
(a) Zhang, S.; Huang, Y.; Kong, L.; Zhang, X.; Yang, J. Dyes Pigm. 2020, 181, 108574;
doi: 10.1016/j.dyepig.2020.108574 |
|
(b) Liu, X.-J.; Hao, J.; Jia, Y. R.; Xia, M. Dyes Pigm. 2020, 172, 107845;
doi: 10.1016/j.dyepig.2019.107845 |
|
|
(c) Ekbote, A.; Han, S. H.; Jadhav, T.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2018, 6, 2077.
doi: 10.1039/C7TC05450E |
|
| [11] |
(a) Sarma, P.; Patir, K.; Sarmah, K. K.; Gogoi, S. K.; Thakuria, R.; Das, P. J. Acta Cryst. 2019, B75, 775;
|
|
(b) Yakir, H. R.; Shimon, L.; Gidron, W. O. Helv. Chim. Acta 2019, 102, e1900027;
doi: 10.1002/hlca.v102.5 |
|
|
(c) Nagura, K.; Saito. S. J. Am. Chem. Soc. 2013, 135, 10322;
doi: 10.1021/ja4055228 |
|
|
(d) Guo, K. P.; Zhang, F.; Guo, S.; Li, K. Chem. Commun. 2017, 53, 1309.
doi: 10.1039/C6CC09186E |
|
| [12] |
(a) Patra, A.; Hebalkar, N.; Sreedhar, B.; Sarkar, M.; Samanta, A.; Radhakrishnan, T. P. Small 2006, 2, 650;
pmid: 23076763 |
|
(b) Chandaluri, C. G.; Radhakrishnan, T. P. Angew. Chem., Int. Ed. 2012, 51, 11849
doi: 10.1002/anie.201205081 pmid: 23076763 |
|
|
(c) Anthony, S. P.; Draper, S. M. J. Phys. Chem. C, 2010, 114, 11708;
doi: 10.1021/jp100594w pmid: 23076763 |
|
|
(d) Molla, M. R.; Gehrig, D.; Roy, L.; Kamm, V.; Paul, A.; Laquai, F.; Ghosh, S. Chem.-Eur. J. 2014, 20, 760;
doi: 10.1002/chem.v20.3 pmid: 23076763 |
|
|
(e) Fernández-Mato, A.; Svnchez-Andújar, M.; Pato-Doldán, B.; Señarís-Rodríguez, M. A.; Platas-Iglesias, C.; Tordera, D.; Bolink, H. J.; Quintela, M.; Peinador, C.; García, M. D. Cryst. Growth Des. 2014, 14, 3849;
doi: 10.1021/cg500379r pmid: 23076763 |
|
|
(f) Fu, H.-Y.; Liu, X.-J.; Xia, M. RSC Adv. 2017, 7, 50720.
doi: 10.1039/C7RA10432D pmid: 23076763 |
| [1] | 刘笑静, 贾彦荣, 江豪, 高贯雷, 夏敏. 三苯胺取代的苯并咪唑的两种晶体:具有不同颜色的摩擦发光与相反发射位移的力致荧光变色[J]. 化学学报, 2019, 77(11): 1194-1202. |
| [2] | 易君明, 肖欣, 张云黔, 薛赛凤, 陶朱, 张建新. 八元瓜环与1,7-二(2-苯并咪唑)-庚烷的超分子自组装[J]. 化学学报, 2014, 72(8): 949-955. |
| [3] | 毛文纲, 陈康, 欧阳密, 孙璟玮, 周永兵, 宋庆宝, 张诚. 基于苯乙烯腈结构的可逆力致变色化合物的合成及性能[J]. 化学学报, 2013, 71(04): 613-618. |
| [4] | 卢艳梅, 区志镔, 胡伟, 乐学义. (2-(2'-吡啶)苯并咪唑)(L-丙氨酸根)铜(II)配合物结构、抗菌活性及DNA断裂作用[J]. 化学学报, 2012, 70(08): 973-979. |
| [5] | 易平贵, 周继明, 于贤勇, 汪朝旭, 李筱芳, 刘峥军, 侯博. 2-(2′-氨基苯基)苯并咪唑衍生物分子内质子转移理论研究: 取代基效应[J]. 化学学报, 2012, 70(06): 699-706. |
| [6] | 岳可芬, 卓飞, 侯磊, 姜严梅, 翟高红, 尹兵, 王尧宇, 文振翼. 1,3-二(1-二茂铁磺酰基-2-苯并咪唑)丙烷的合成、单晶结构和量子化学研究[J]. 化学学报, 2011, 69(05): 596-600. |
| [7] | 谢湖均, 雷群芳, 胡晓环, 宣贵达, 方文军. 1-取代-2-氨基苯并咪唑衍生物的理论计算及QSAR研究[J]. 化学学报, 2011, 69(04): 399-404. |
| [8] | 陈战国, 王传宁, 赵朋飞, 王芸, 周利燕. 2-甲基苯并咪唑基异黄酮衍生物的合成及抗氧化活性[J]. 化学学报, 2010, 68(22): 2347-2355. |
| [9] | 岳可芬, 姜严梅, 宋扬, 姜富灵, 黄小英, 李涛, 王诚军, 王尧宇. 一个“囍”字配合物的合成、晶体结构、量化计算及抑菌活性[J]. 化学学报, 2010, 68(13): 1291-1297. |
| [10] | . 由二茂铁基羧酸以及苯并咪唑基配体构筑的两个镉(II)配合物的合成、晶体结构及性质[J]. 化学学报, 2009, 67(9): 1026-1031. |
| [11] | 易平贵, 王涛, 周继明, 彭洪亮, 李筱芳, 于贤勇, 汪朝旭. 2-(3-羟基-2-吡啶基)-苯并咪唑的合成、晶体结构及光谱研究[J]. 化学学报, 2009, 67(24): 2803-2808. |
| [12] | 朱莉,于贤勇,龙云飞,林原斌. 2-苯并咪唑吡啶镍配合物的合成﹑晶体结构及与DNA相互作用的共振散射光谱研究[J]. 化学学报, 2009, 67(2): 139-144. |
| [13] | 李庆祥,沈云军,孟祥高. 1-亚甲基苯并咪唑-1,4,7-三氮环壬烷配体及其铜配合物[Cu(C14H21N5)Br]2•[CuBr4]的合成与晶体结构[J]. 化学学报, 2008, 66(2): 266-270. |
| [14] | 冯少波a 张 业b 周 琪a 李芳耀c 潘英明a 陈振锋a 王恒山,a. 新型脱氢松香基苯并咪唑类衍生物的合成及其氯离子识别性能[J]. 化学学报, 2008, 66(12): 1490-1496. |
| [15] | 闫振忠, 唐瑜, 谭民裕, 刘伟生, 王大奇. 2,6-二(2-苯并咪唑基)吡啶与稀土苦味酸盐配合物的合成、晶体结构及荧光性质研究[J]. 化学学报, 2007, 65(7): 607-614. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||