化学学报 ›› 2024, Vol. 82 ›› Issue (5): 477-485.DOI: 10.6023/A24020054 上一篇 下一篇
研究论文
投稿日期:2024-02-17
发布日期:2024-04-07
基金资助:
Hao Liu†, Xuli Xu†, Yong Guo, Xiaohui Liu( ), Yanqin Wang
), Yanqin Wang
Received:2024-02-17
Published:2024-04-07
Contact:
*E-mail: xhliu@ecust.edu.cn
About author:Supported by:文章分享

电催化还原胺化是一种环境友好且可持续的合成胺途径. 本工作建立了一个以2Ru/NiPOx为阴极催化苯甲醛和环己胺电化学还原胺化反应的体系, 该体系可以高效获得95.1%的N-苄基环己胺产率, 以及64.7%的法拉第效率, 其中活化二甲基亚砜(DMSO)还原胺化与DMSO自身反应之间具有相匹配的活性是实现定向氢转移还原胺化的关键. 最后, 通过漫反射红外傅里叶变换光谱(DRIFTS)表征、自由基捕获实验和DMSO-D6同位素标记实验探索了反应的机理.
刘浩, 徐旭莉, 郭勇, 刘晓晖, 王艳芹. Ru/NiPOx高效电催化醛还原胺化反应的研究[J]. 化学学报, 2024, 82(5): 477-485.
Hao Liu, Xuli Xu, Yong Guo, Xiaohui Liu, Yanqin Wang. Efficient Electro-catalytic Reductive Amination of Aldehyde over Ru Deposited on Nickel Phosphate[J]. Acta Chimica Sinica, 2024, 82(5): 477-485.


| 催化剂 | 2b产率/% | FE/% |
|---|---|---|
| 2Ru/NiPOx | 95.1 | 64.7 |
| 2Ru/Nb2O5 | 82.9 | 54.4 |
| 2Ru/Co3O4 | 87.2 | 51.5 |
| 2Ru/Al2O3 | 86.0 | 60.3 |
| 2Ru/C | 76.4 | 50.6 |
| CP | 65.6 | 59.0 |
| 催化剂 | 2b产率/% | FE/% |
|---|---|---|
| 2Ru/NiPOx | 95.1 | 64.7 |
| 2Ru/Nb2O5 | 82.9 | 54.4 |
| 2Ru/Co3O4 | 87.2 | 51.5 |
| 2Ru/Al2O3 | 86.0 | 60.3 |
| 2Ru/C | 76.4 | 50.6 |
| CP | 65.6 | 59.0 |
| 催化剂 | 2b产率/% | FE/% |
|---|---|---|
| NiPOx | 84.7 | 56.5 |
| 0.5Ru/NiPOx | 87.5 | 60.8 |
| 1Ru/NiPOx | 88.5 | 63.1 |
| 2Ru/NiPOx | 95.1 | 64.7 |
| 5Ru/NiPOx | 97.0 | 57.8 |
| 催化剂 | 2b产率/% | FE/% |
|---|---|---|
| NiPOx | 84.7 | 56.5 |
| 0.5Ru/NiPOx | 87.5 | 60.8 |
| 1Ru/NiPOx | 88.5 | 63.1 |
| 2Ru/NiPOx | 95.1 | 64.7 |
| 5Ru/NiPOx | 97.0 | 57.8 |
| 溶剂 | 2b产率/% | FE/% |
|---|---|---|
| DMF | 74.7 | 52.3 |
| MeOH | 44.3 | 5.6 |
| Acetone | 27.5 | 10.3 |
| Toluene | — | — |
| p-Xylene | — | — |
| THF | — | — |
| 溶剂 | 2b产率/% | FE/% |
|---|---|---|
| DMF | 74.7 | 52.3 |
| MeOH | 44.3 | 5.6 |
| Acetone | 27.5 | 10.3 |
| Toluene | — | — |
| p-Xylene | — | — |
| THF | — | — |
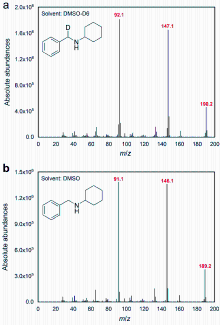
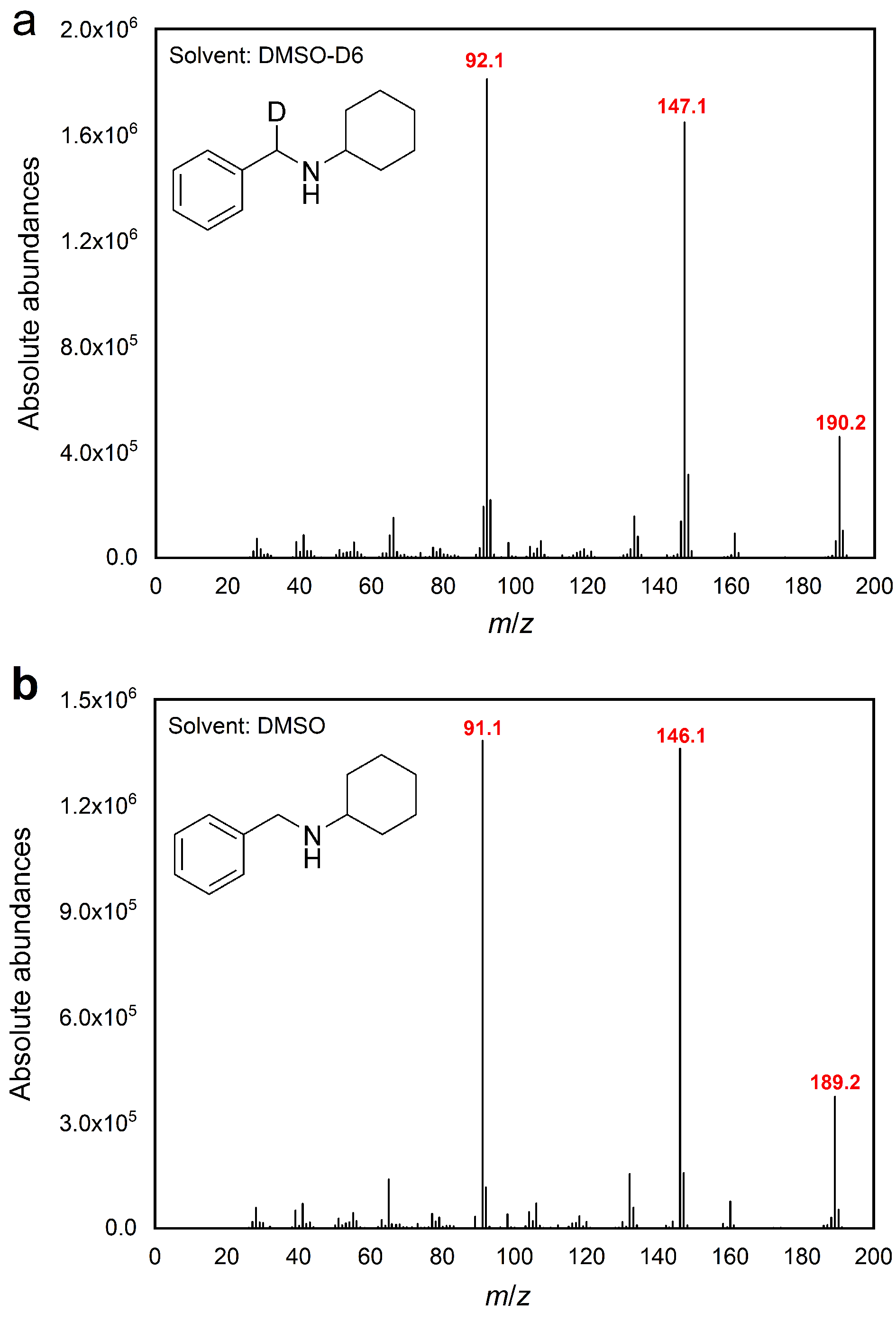
| [1] |
Liu, J.; Ou, J.; Li, Z.; Jiang, J.; Liang, R.; Zhang, W.; Liu, K.; Han, Y. Acta Chim. Sinica 2023, 81, 1701 (in Chinese).
doi: 10.6023/A23080374 |
|
(刘健, 欧金花, 李泽平, 蒋婧怡, 梁荣涛, 张文杰, 刘开建, 韩瑜, 化学学报, 2023, 81, 1701.)
doi: 10.6023/A23080374 |
|
| [2] |
Gomez, S.; Peters, J. A.; Maschmeyer, T. Adv. Synth. Catal. 2002, 344, 1037.
|
| [3] |
Guo, X.; Okamoto, Y.; Schreier, M. R.; Ward, T. R.; Wenger, O. S. Eur. J. Org. Chem. 2020, 2020, 1288.
|
| [4] |
Trowbridge, A.; Walton, S. M.; Gaunt, M. J. Chem. Rev. 2020, 120, 2613.
doi: 10.1021/acs.chemrev.9b00462 pmid: 32064858 |
| [5] |
Wang, A. Z.; Liang, Y.-Y.; Zheng, J.-S. Curr. Org. Synth. 2018, 15, 755.
|
| [6] |
Hou, Z.-W.; Liu, D.-J.; Xiong, P.; Lai, X.-L.; Song, J.; Xu, H.-C. Angew. Chem., Int. Ed. 2021, 60, 2943.
|
| [7] |
Hou, Z.-W.; Li, L.; Wang, L. Org. Chem. Front. 2021, 8, 4700.
|
| [8] |
Ruan, Z.; Huang, Z.; Xu, Z.; Zeng, S.; Feng, P.; Sun, P.-H. Sci. China: Chem. 2021, 64, 800.
|
| [9] |
Liu, C.; Liu, J.; Li, W.; Lu, H.; Zhang, Y. Org. Chem. Front. 2023, 10, 5309.
|
| [10] |
He, Y.; Teng, J.; Tian, C.; Borzov, M.; Hu, Q.; Nie, W. Acta Chim. Sinica 2018, 76, 774 (in Chinese).
|
|
(何云清, 滕金伟, 田冲, Borzov, Maxima, 胡启山, 聂万丽, 化学学报, 2018, 76, 774.)
doi: 10.6023/A18070281 |
|
| [11] |
Tripathi, P. R.; Verma, S. S.; Pandey, J.; Tiwari, K. V. Curr. Org. Chem. 2008, 12, 1093.
|
| [12] |
Hahn, G.; Kunnas, P.; de Jonge, N.; Kempe, R. Nat. Catal. 2019, 2, 71.
doi: 10.1038/s41929-018-0202-6 |
| [13] |
Senthamarai, T.; Murugesan, K.; Schneidewind, J.; Kalevaru, N. V.; Baumann, W.; Neumann, H.; Kamer, P. C. J.; Beller, M.; Jagadeesh, R. V. Nat. Commun. 2018, 9, 4123.
|
| [14] |
Murugesan, K.; Beller, M.; Jagadeesh, R. V. Angew. Chem., Int. Ed. 2019, 58, 5064.
|
| [15] |
Roylance, J. J.; Choi, K. S. Green Chem. 2016, 18, 5412.
|
| [16] |
Schiffer, Z. J.; Chung, M.; Steinberg, K.; Manthiram, K. Chem Catal. 2023, 3, 100500.
|
| [17] |
Borch, R. F.; Bernstein, M. D.; Durst, H. D. J. Am. Chem. Soc. 1971, 93, 2897.
|
| [18] |
Saberi, D.; Akbari, J.; Mahdudi, S.; Heydari, A. J. Mol. Liq. 2014, 196, 208.
|
| [19] |
Fasano, V.; Radcliffe, J. E.; Ingleson, M. J. ACS Catal. 2016, 6, 1793.
|
| [20] |
Takale, B. S.; Feng, X.; Lu, Y.; Bao, M.; Jin, T.; Minato, T.; Yamamoto, Y. J. Am. Chem. Soc. 2016, 138, 10356.
|
| [21] |
Hoshimoto, Y.; Kinoshita, T.; Hazra, S.; Ohashi, M.; Ogoshi, S. J. Am. Chem. Soc. 2018, 140, 7292.
doi: 10.1021/jacs.8b03626 pmid: 29790343 |
| [22] |
Hong, H.; Zou, Z.; Liang, G.; Pu, S.; Hu, J.; Chen, L.; Zhu, Z.; Li, Y.; Huang, Y. Org. Biomol. Chem. 2020, 18, 5832.
|
| [23] |
Kim, T.; Park, D. I.; Kim, S.; Yadav, D.; Hong, S.; Kim, S. H.; Yoon, H. J.; Jin, K. Chem. Commun. 2023, 59, 4818.
|
| [24] |
Jiang, Y.; Xu, K.; Zeng, C. Chem. Rev. 2018, 118, 4485.
|
| [25] |
Xiong, P.; Long, H.; Song, J.; Wang, Y.; Li, J.-F.; Xu, H.-C. J. Am. Chem. Soc. 2018, 140, 16387.
doi: 10.1021/jacs.8b08592 pmid: 30384602 |
| [26] |
Li, J.; He, L.; Liu, X.; Cheng, X.; Li, G. Angew. Chem., Int. Ed. 2019, 58, 1759.
|
| [27] |
Fokin, I.; Siewert, I. Chem. - Eur. J. 2020, 26, 14137.
|
| [28] |
Huang, B.; Li, Y.; Yang, C.; Xia, W. Chem. Commun. 2019, 55, 6731.
|
| [29] |
Murtz, S. D.; Kurig, N.; Holzhauser, F. J.; Palkovits, R. Green Chem. 2021, 23, 8428.
|
| [30] |
Fang, S.; Zhong, K.; Zeng, S.; Hu, X.; Sun, P.; Ruan, Z. Chem. Commun. 2023, 59, 11425.
|
| [31] |
Sun, M.; Zhou, Y.; Li, L.; Wang, L.; Ma, Y.; Li, P. Org. Chem. Front. 2021, 8, 754.
|
| [32] |
Deng, D.; Kita, Y.; Kamata, K.; Hara, M. ACS Sustainable Chem. Eng. 2018, 7, 4692.
|
| [33] |
Li, B.; Liu, S.; Lin, Q.; Shao, Y.; Peng, S.; Li, Y. Chem. Commun. 2018, 54, 9214.
|
| [34] |
Nishimura, S.; Mizuhori, K.; Ebitani, K. Res. Chem. Intermed. 2016, 42, 19.
|
| [35] |
Bastakoti, B. P.; Munkaila, S.; Guragain, S. Mater. Lett. 2019, 251, 34.
doi: 10.1016/j.matlet.2019.05.034 |
| [36] |
O'Brien, A. G.; Maruyama, A.; Inokuma, Y.; Fujita, M.; Baran, P. S. Angew. Chem., Int. Ed. 2014, 53, 11868.
|
| [37] |
Sauer, G. S.; Lin, S. ACS Catal. 2018, 8, 5175.
|
| [38] |
Bijaya, B. K.; Lingden, C. P.; Pokhrel, T.; Paudel, M.; Sajid, K.; Adhikari, A.; Shirinfar, B.; Ahmed, N. ChemElectroChem 2023, 10, e202300289.
|
| [39] |
Akbar, S.; Beyou, E.; Chaumont, P.; Melis, F. Macromol. Chem. Phys. 2010, 211, 2396.
|
| [40] |
Vovk, A. I.; Shivanyuk, A. M.; Bugas, R. V.; Muzychka, O. V.; Melnyk, A. K. Bioorg. Med. Chem. Lett. 2009, 19, 1314.
|
| [1] | 赵勇, 李施宏, 张苗苗, 刘峰. 氨基酸酯Katritzky盐用于β,γ-不饱和酯和γ-酮酯合成的研究[J]. 化学学报, 2019, 77(9): 916-921. |
| [2] | 顾正洋, 纪顺俊. 钴催化异腈参与的偶联反应研究进展[J]. 化学学报, 2018, 76(5): 347-356. |
| [3] | 陈栋, 吉梅山, 姚英明, 朱晨. 通过远端碳氮双键迁移实现非活化烯烃的三氟甲硫基化反应[J]. 化学学报, 2018, 76(12): 951-955. |
| [4] | 张晶, 陈以昀. 可见光引发的氧自由基的新型产生方式及反应[J]. 化学学报, 2017, 75(1): 41-48. |
| [5] | 王德红, 张龙, 罗三中. 催化不对称光诱导自由基反应[J]. 化学学报, 2017, 75(1): 22-33. |
| [6] | 余宽, 高北岭, 丁寒锋. 吲哚生物碱(+)-Alsmaphorazine D的不对称全合成及其绝对构型更正[J]. 化学学报, 2016, 74(5): 410-414. |
| [7] | 张令, 张沛之, 薛剑飞, 孙网彬, 邹建平. 醋酸锰引发的吲哚膦酰化反应[J]. 化学学报, 2016, 74(10): 811-818. |
| [8] | 王业红, 谭涓, 刘靖, 陈颖, 李旭影. 萃取法脱除介孔磷酸镍模板剂的研究[J]. 化学学报, 2010, 68(23): 2471-2476. |
| [9] | 张一平,费金华, 于英民, 郑小明. 固载Ru基催化剂上二氧化碳加氢合成甲酸的研究(IV): 反应机理[J]. 化学学报, 2007, 65(4): 289-294. |
| [10] | 张一平,费金华,于英民,郑小明. 二氧化硅固载Ru基催化剂上二氧化碳加氢合成甲酸的研究(III): 配体对催化剂反应性能的影响[J]. 化学学报, 2006, 64(9): 845-850. |
| [11] | 孙翠红,曾艳丽,孟令鹏,郑世钧. Cl原子与CH2SH自由基反应机理及电子密度拓扑研究[J]. 化学学报, 2005, 63(4): 295-300. |
| [12] | 闫革新, 刘伟平, 高文桂, 胡昌义, 余巍, 梁广. 三(2,4-戊二酮)合铱(Ⅲ)的热分解行为[J]. 化学学报, 2004, 62(19): 1901-1906. |
| [13] | 丁元庆, 王超, 方德彩, 刘若庄. 二重态下反应HCCO(2A″)+O2(3∑g-)的势能面理论研究[J]. 化学学报, 2004, 62(15): 1373-1378. |
| [14] | 陈德展,杨仲年,王道平,孟琳. F+CH_3OH碰撞反应机机理和反应势能面[J]. 化学学报, 2003, 61(8): 1213-1219. |
| [15] | 李来才,田安民,徐明厚. CH_2CH(~2A')自由基与臭氧反应机理的理论研究[J]. 化学学报, 2003, 61(8): 1256-1260. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||