化学学报 ›› 2024, Vol. 82 ›› Issue (12): 1234-1240.DOI: 10.6023/A24090276 上一篇 下一篇
研究论文
许木榕a, 周纯b, 王子慧a, 杨丽冰a, 李晨远a, 卓炜丰a, 王行之b,*( ), 杜克钊a,*(
), 杜克钊a,*( )
)
投稿日期:2024-09-12
发布日期:2024-12-01
基金资助:
Murong Xua, Chun Zhoub, Zihui Wanga, Libing Yanga, Chenyuan Lia, Weifeng Zhuoa, Xingzhi Wangb( ), Kezhao Dua(
), Kezhao Dua( )
)
Received:2024-09-12
Published:2024-12-01
Contact:
E-mail: Supported by:文章分享
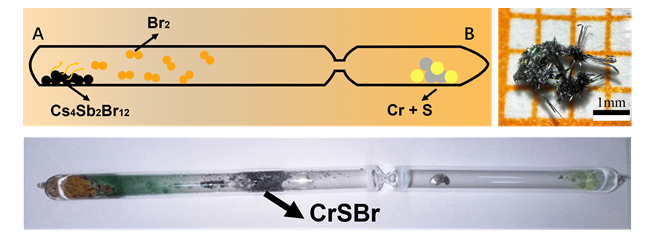
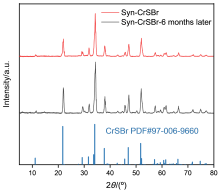
CrSBr是一种磁性二维材料, 因其独特的磁学、电学和光学特性而受到越来越多的关注. 然而, CrSBr晶体的传统合成方法都需要以液溴作为原料, 而液溴具有挥发性、毒性和腐蚀性, 为CrSBr晶体的合成带来了不便与危险. 为了解决该问题, 本工作在尝试多种溴素储存晶体后, 选择具有化学固溴性能的Cs4Sb2Br12化合物作为新溴源, 取代液溴, 成功合成了CrSBr晶体. 通过X射线衍射(XRD)、光致发光(PL)和拉曼(Raman)光谱等手段对合成的CrSBr晶体进行了表征. 本工作开发的固态溴素合成法, 为CrSBr晶体的合成提供了更好的安全性, 也为其他固态卤素在晶体合成中的应用提供了新的思路.
许木榕, 周纯, 王子慧, 杨丽冰, 李晨远, 卓炜丰, 王行之, 杜克钊. 固态溴素合成溴化物晶体的研究——以CrSBr合成和表征为例[J]. 化学学报, 2024, 82(12): 1234-1240.
Murong Xu, Chun Zhou, Zihui Wang, Libing Yang, Chenyuan Li, Weifeng Zhuo, Xingzhi Wang, Kezhao Du. Solid-State Bromine for Bromide Synthesis, a Case Study of CrSBr[J]. Acta Chimica Sinica, 2024, 82(12): 1234-1240.
| 序号 | 溴源 | 管子长度 | 条件a | 实验结果 | 是否成功 | 备注 |
|---|---|---|---|---|---|---|
| 1 | Cs4Sb2Br12 | 24 cm | 溴源端分开 | CrSBr | √ | 针状黑色晶体产出 |
| 2 | Cs4Sb2Br12 | 10 cm | 溴源端分开 | Cr0.67S | × | 两端温差为100 ℃, 不利于CrSBr合成 |
| 3 | Cs4Sb2Br12 | 24 cm | 溴源端未分开 | Cr0.67S | × | 无法较好释放溴素 |
| 4 | KBrO3+KBr | 24 cm | 溴源端未分开 | 爆管 | × | 原料对石英管具有一定腐蚀性 |
| 5 | KBrO3+KBr | 24 cm | 溴源端未分开, 镀膜b | Cr2O3 | × | KBrO3高温分解, 提供溴源不稳定 |
| 6 | CsBr3 | — | 溴源端未分开 | — | × | CsBr3不稳定, 未封管已变为白色CsBr |
| 7 | C16H36NBr3 | 13 cm | 溴源端未分开 | 爆管 | × | 有机物碳化, 导致气压上升 |
| 序号 | 溴源 | 管子长度 | 条件a | 实验结果 | 是否成功 | 备注 |
|---|---|---|---|---|---|---|
| 1 | Cs4Sb2Br12 | 24 cm | 溴源端分开 | CrSBr | √ | 针状黑色晶体产出 |
| 2 | Cs4Sb2Br12 | 10 cm | 溴源端分开 | Cr0.67S | × | 两端温差为100 ℃, 不利于CrSBr合成 |
| 3 | Cs4Sb2Br12 | 24 cm | 溴源端未分开 | Cr0.67S | × | 无法较好释放溴素 |
| 4 | KBrO3+KBr | 24 cm | 溴源端未分开 | 爆管 | × | 原料对石英管具有一定腐蚀性 |
| 5 | KBrO3+KBr | 24 cm | 溴源端未分开, 镀膜b | Cr2O3 | × | KBrO3高温分解, 提供溴源不稳定 |
| 6 | CsBr3 | — | 溴源端未分开 | — | × | CsBr3不稳定, 未封管已变为白色CsBr |
| 7 | C16H36NBr3 | 13 cm | 溴源端未分开 | 爆管 | × | 有机物碳化, 导致气压上升 |








| 试剂名称 | 化学式 | 纯度 | 生产厂家 |
|---|---|---|---|
| 溴化铯 三氧化二锑 溴酸钾 氢溴酸 铬 硫 | CsBr Sb2O3 KBrO3 HBr Cr S | 99.9% 99% 99% 40%~48% 99.95% 99.99% | Adamas Acros Aladdin Aladdin Adamas Adamas |
| 试剂名称 | 化学式 | 纯度 | 生产厂家 |
|---|---|---|---|
| 溴化铯 三氧化二锑 溴酸钾 氢溴酸 铬 硫 | CsBr Sb2O3 KBrO3 HBr Cr S | 99.9% 99% 99% 40%~48% 99.95% 99.99% | Adamas Acros Aladdin Aladdin Adamas Adamas |
| [1] |
Wang, Q. H.; Bedoya-Pinto, A.; Blei, M.; Dismukes, A. H.; Hamo, A.; Jenkins, S.; Koperski, M.; Liu, Y.; Sun, Q. C.; Telford, E. J.; Kim, H. H.; Augustin, M.; Vool, U.; Yin, J. X.; Li, L. H.; Falin, A.; Dean, C. R.; Casanova, F.; Evans, R. F. L.; Chshiev, M.; Mishchenko, A.; Petrovic, C.; He, R.; Zhao, L.; Tsen, A. W.; Gerardot, B. D.; Brotons-Gisbert, M.; Guguchia, Z.; Roy, X.; Tongay, S.; Wang, Z.; Hasan, M. Z.; Wrachtrup, J.; Yacoby, A.; Fert, A.; Parkin, S.; Novoselov, K. S.; Dai, P.; Balicas, L.; Santos, E. J. G. ACS Nano 2022, 16, 6960.
|
| [2] |
Ziebel, M. E.; Feuer, M. L.; Cox, J.; Zhu, X.; Dean, C. R.; Roy, X. Nano Lett 2024, 24, 4319.
|
| [3] |
Dirnberger, F.; Quan, J.; Bushati, R.; Diederich, G. M.; Florian, M.; Klein, J.; Mosina, K.; Sofer, Z.; Xu, X.; Kamra, A.; García-Vidal, F. J.; Alù, A.; Menon, V. M. Nature 2023, 620, 533.
|
| [4] |
Bae, Y.; Wang, J.; Scheie, A.; Xu, J.-W.; Chica, D. G.; Diederich, G.; Cenker, J.; Ziebel, M. E.; Bai, Y.; Ren, H.; Dean, C.; Delor, M.; Xu, X.; Roy, X.; Kent, A.; Zhu, X. Nature 2022, 609, 282.
|
| [5] |
Boix-Constant, C.; Jenkins, S.; Rama-Eiroa, R.; Santos, E. J. G.; Mañas-Valero, S.; Coronado, E. Nat. Mater. 2023, 23, 212.
|
| [6] |
Burch, K. S.; Mandrus, D.; Park, J.-G. Nature 2018, 563, 47.
|
| [7] |
Gong, C.; Zhang, X. Science 2019, 363, 706.
|
| [8] |
Tabataba-Vakili, F.; Nguyen, H. P. G.; Rupp, A.; Mosina, K.; Papavasileiou, A.; Watanabe, K.; Taniguchi, T.; Maletinsky, P.; Glazov, M. M.; Sofer, Z.; Baimuratov, A. S.; Högele, A. Nat. Commun. 2024, 15, 4735.
doi: 10.1038/s41467-024-49048-9 pmid: 38830857 |
| [9] |
Tschudin, M. A.; Broadway, D. A.; Siegwolf, P.; Schrader, C.; Telford, E. J.; Gross, B.; Cox, J.; Dubois, A. E. E.; Chica, D. G.; Rama-Eiroa, R.; Santos, E. J. G.; Poggio, M.; Ziebel, M. E.; Dean, C. R.; Roy, X.; Maletinsky, P. Nat. Commun. 2024, 15, 6005.
doi: 10.1038/s41467-024-49717-9 pmid: 39019853 |
| [10] |
Beck, J. Z. Anorg. Allg. Chem. 1990, 585, 157.
|
| [11] |
Lopez-Paz, S. A.; Guguchia, Z.; Pomjakushin, V. Y.; Witteveen, C.; Cervellino, A.; Luetkens, H.; Casati, N.; Morpurgo, A. F.; von Rohr, F. O. Nat. Commun. 2022, 13, 4745.
|
| [12] |
Klein, J.; Pham, T.; Thomsen, J. D.; Curtis, J. B.; Denneulin, T.; Lorke, M.; Florian, M.; Steinhoff, A.; Wiscons, R. A.; Luxa, J.; Sofer, Z.; Jahnke, F.; Narang, P.; Ross, F. M. Nat. Commun. 2022, 13, 5420.
doi: 10.1038/s41467-022-32737-8 pmid: 36109520 |
| [13] |
Long, F.; Mosina, K.; Hübner, R.; Sofer, Z.; Klein, J.; Prucnal, S.; Helm, M.; Dirnberger, F.; Zhou, S. Appl. Phys. Lett. 2023, 123, 222401.
|
| [14] |
Scheie, A.; Ziebel, M.; Chica, D. G.; Bae, Y. J.; Wang, X.; Kolesnikov, A. I.; Zhu, X.; Roy, X. J. A. S. 2022, 9, 2202467.
|
| [15] |
Lin, H.; Ma, R.; Jiang, Y.; Xu, M.; Lin, Y.; Du, K. Acta Chim. Sinica 2024, 82, 62. (in Chinese)
|
|
(林航青, 马若茹, 江怡蓝, 许木榕, 林洋彭, 杜克钊, 化学学报, 2024, 82, 62.)
doi: 10.6023/A23080392 |
|
| [16] |
Lin, Y.-P.; Huang, X.-Y.; Du, K.-Z. Mater. Chem. Phys. 2022, 280, 125820.
|
| [17] |
Lin, Y. P.; Xia, B.; Hu, S.; Zhong, Y.; Huang, Y. E.; Zhang, Z. Z.; Wu, N.; Wu, Y. W.; Wu, X. H.; Huang, X. Y.; Xiao, Z.; Du, K. Z. Energy Environ. Mater. 2020, 3, 535.
|
| [18] |
Lin, K.; Li, Y.; Ghorbani-Asl, M.; Sofer, Z.; Winnerl, S.; Erbe, A.; Krasheninnikov, A.; Helm, M.; Zhou, S.; Dan, Y.; Prucnal, S. J. Phys. Chem. Lett. 2024, 15, 6010.
|
| [19] |
Wilson, N. P.; Lee, K.; Cenker, J.; Xie, K.; Dismukes, A. H.; Telford, E. J.; Fonseca, J.; Sivakumar, S.; Dean, C.; Cao, T.; Roy, X.; Xu, X.; Zhu, X. Nat. Mater. 2021, 20, 1657.
|
| [20] |
Pawbake, A.; Pelini, T.; Wilson, N.; Mosina, K.; Sofer, Z.; Heid, R.; Faugeras, C. Phys. Rev. B 2023, 107, 075421.
|
| [21] |
Torres, K.; Kuc, A.; Maschio, L.; Pham, T.; Reidy, K.; Dekanovsky, L.; Sofer, Z.; Ross, F. M.; Klein, J. Adv. Funct. Mater. 2023, 33, 2211366.
|
| [22] |
Lin, K.; Sun, X.; Dirnberger, F.; Li, Y.; Qu, J.; Wen, P.; Sofer, Z.; Söll, A.; Winnerl, S.; Helm, M.; Zhou, S.; Dan, Y.; Prucnal, S. ACS Nano 2024, 18, 2898.
|
| [23] |
Klein, J.; Song, Z.; Pingault, B.; Dirnberger, F.; Chi, H.; Curtis, J. B.; Dana, R.; Bushati, R.; Quan, J.; Dekanovsky, L.; Sofer, Z.; Alu, A.; Menon, V. M.; Moodera, J. S.; Loncar, M.; Narang, P.; Ross, F. M. ACS Nano 2023, 17, 288.
|
| [24] |
Klein, J.; Pingault, B.; Florian, M.; Heißenbüttel, M.-C.; Steinhoff, A.; Song, Z.; Torres, K.; Dirnberger, F.; Curtis, J. B.; Weile, M.; Penn, A.; Deilmann, T.; Dana, R.; Bushati, R.; Quan, J.; Luxa, J.; Sofer, Z.; Alù, A.; Menon, V. M.; Wurstbauer, U.; Rohlfing, M.; Narang, P.; Lončar, M.; Ross, F. M. ACS Nano 2023, 17, 5316.
|
| [25] |
Marques-Moros, F.; Boix-Constant, C.; Mañas-Valero, S.; Canet-Ferrer, J.; Coronado, E. ACS Nano 2023, 17, 13224.
doi: 10.1021/acsnano.3c00375 pmid: 37442121 |
| [1] | 刘卫涛, 高战胜, 黄明举, 刘忠范, 陈珂. 共形二维材料: 创制与应用[J]. 化学学报, 2024, 82(11): 1162-1179. |
| [2] | 王娟, 肖华敏, 谢丁, 郭元茹, 潘清江. 铜掺杂与氮化碳复合氧化锌材料结构和二氧化氮气体传感性质的密度泛函理论计算[J]. 化学学报, 2023, 81(11): 1493-1499. |
| [3] | 张芬, 李霄琪, 韩世国, 邬发发, 刘希涛, 孙志华, 罗军华. 大尺寸二维卤化物钙钛矿铁电晶体的生长及偏振光电探测性能研究※[J]. 化学学报, 2022, 80(3): 237-243. |
| [4] | 周家正, 徐啸, 段碧雯, 石将建, 罗艳红, 吴会觉, 李冬梅, 孟庆波. 铜锌锡硫硒薄膜太阳能电池一价金属替位的研究进展[J]. 化学学报, 2021, 79(3): 303-318. |
| [5] | 陈钱, 匡勤, 谢兆雄. 二维材料在光催化二氧化碳还原中的研究进展[J]. 化学学报, 2021, 79(1): 10-22. |
| [6] | 王文彬, 温群磊, 刘友文, 翟天佑. 表界面化学调控二维材料电催化生物质转化的研究进展[J]. 化学学报, 2020, 78(11): 1185-1199. |
| [7] | 李绍周, 黄晓, 张华. 有机或金属-有机二维纳米材料的制备与应用[J]. 化学学报, 2015, 73(9): 913-923. |
| [8] | 何学侠, 刘富才, 曾庆圣, 刘政 . 二维材料双电层场晶体管的研究[J]. 化学学报, 2015, 73(9): 924-935. |
| [9] | 王璐, 高峻峰, 丁峰. 经典晶体生长理论在石墨烯CVD成核和连续生长中的应用[J]. 化学学报, 2014, 72(3): 345-358. |
| [10] | 段俐,康琦,李根培. 溶菌酶晶体生长过程中的扩散传质问题[J]. 化学学报, 2009, 67(4): 307-312. |
| [11] | 戴国亮, 彭玲, 解莹, 康琦, 胡文瑞. 分子间相互作用对蛋白质晶体生长的影响[J]. 化学学报, 2007, 65(17): 1767-1772. |
| [12] | 于泳,陈万春,康琦,刘道丹,戴国亮,崔海亮. 配液结晶法制备溶菌酶蛋白质晶体的生长机理研究[J]. 化学学报, 2006, 64(12): 1284-1290. |
| [13] | 戴国亮,代连花,于泳,谢莹. 异硫氰酸荧光素-溶菌酶的制备及晶体生长预研究[J]. 化学学报, 2005, 63(7): 559-561. |
| [14] | 戴国亮, 于泳, 康琦, 胡文瑞. 溶菌酶晶体生长前期溶液中聚集体研究[J]. 化学学报, 2004, 62(8): 757-761. |
| [15] | 戴国亮,胡文瑞. NaCl对液-液扩散法生长溶菌酶晶体的影响[J]. 化学学报, 2003, 61(4): 520-525. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||