化学学报 ›› 2025, Vol. 83 ›› Issue (4): 360-368.DOI: 10.6023/A24110355 上一篇 下一篇
研究论文
投稿日期:2024-11-25
发布日期:2025-03-12
基金资助:
Lingling Ma, Lin Ling, Yaming Wu, Yuxue Li( ), Long Lu(
), Long Lu( )
)
Received:2024-11-25
Published:2025-03-12
Contact:
E-mail: Supported by:文章分享

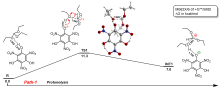
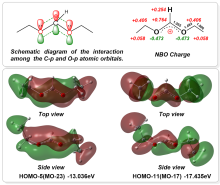
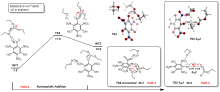
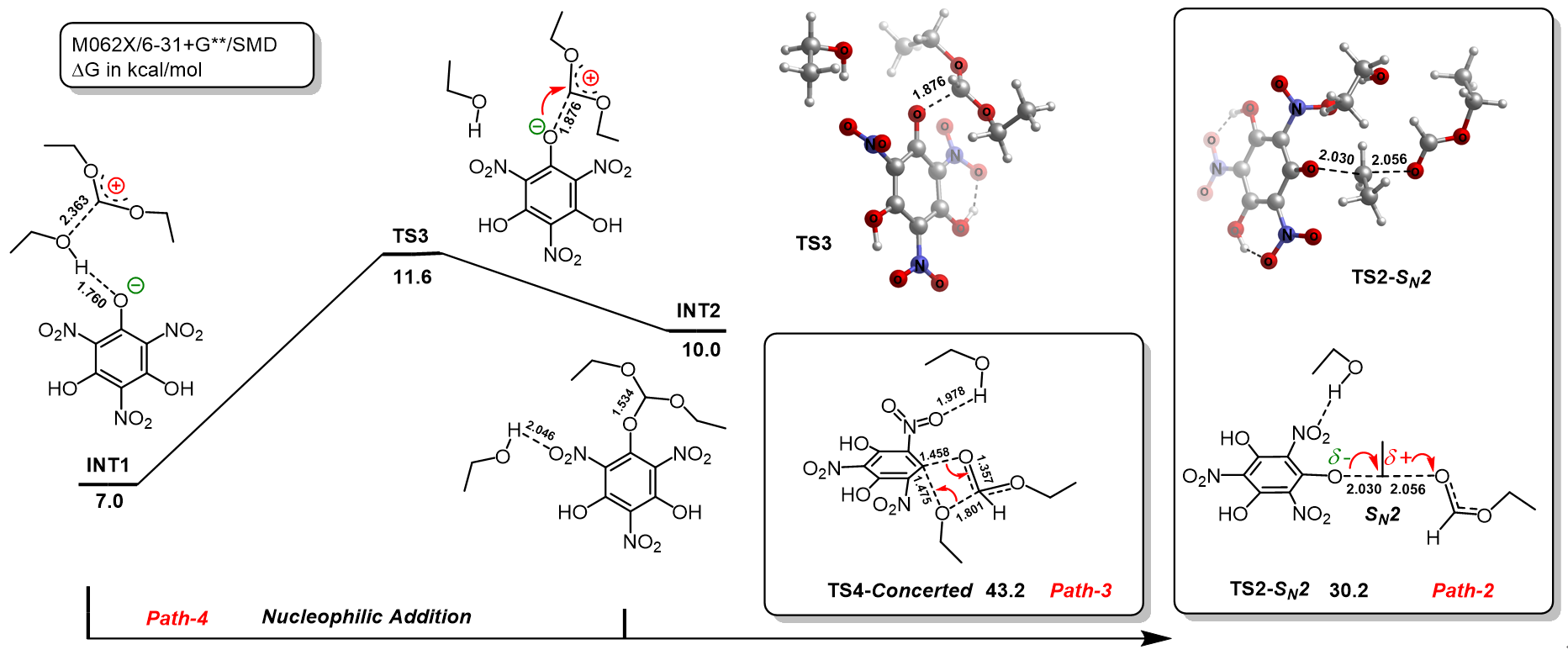
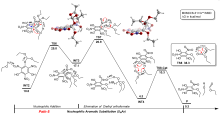
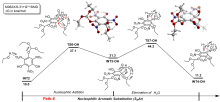
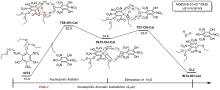
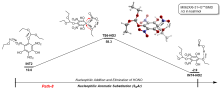
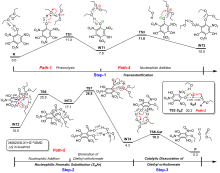
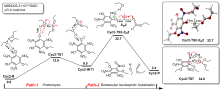
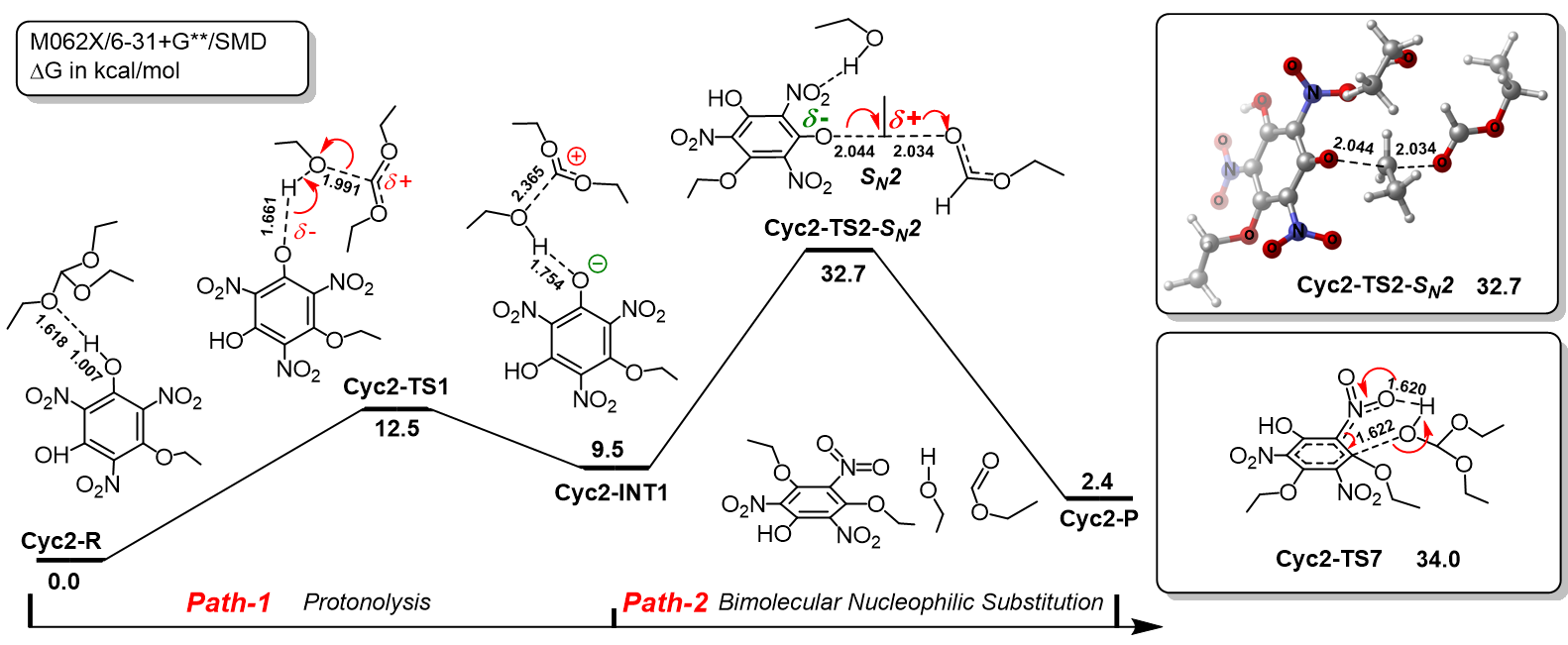
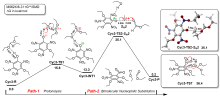
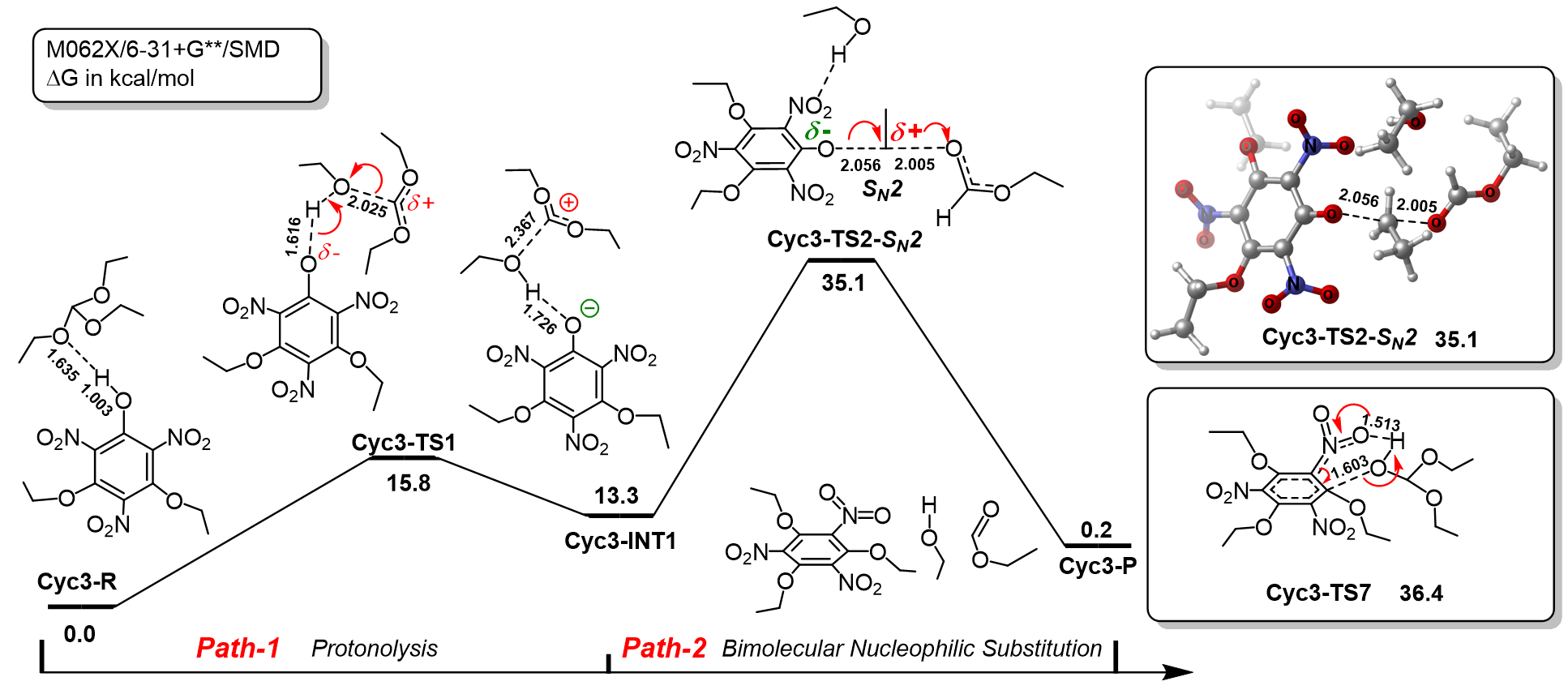
三氨基三硝基苯(TATB)具有出色的安定性, 机械感度极低, 作为重要组分应用于核武器的高聚物黏结炸药(PBX). 然而, 目前工业制备TATB的主要方法存在效率低、杂质残留等问题. 基于以间苯三酚为原料的TATB的新合成路线, 我们发明了一种连续化高效制备TATB前体三乙氧基三硝基苯(TETNB)的方法. 鉴于该反应的重要性, 在M062X/6-31+G**水平上对其反应机理进行了深入的理论研究, 阐明了原甲酸三乙酯作为烷基化试剂进行烷基化反应的详细反应机理, 反应的关键中间体是二乙氧基碳正离子, 关键反应过程是双分子亲核取代(SN2)和芳香亲核取代(SNAr). 研究发现, 和反应物相比, 醚化产物在热力学上是稍微不利的, 达到平衡时产物所占比例较小. 因此, 实验中要不断移除副产物乙醇和甲酸乙酯, 拉动平衡向产物方向移动.
马玲玲, 凌琳, 吴亚明, 李玉学, 吕龙. 1,3,5-三乙氧基-2,4,6-三硝基苯的连续化制备反应机理的理论研究[J]. 化学学报, 2025, 83(4): 360-368.
Lingling Ma, Lin Ling, Yaming Wu, Yuxue Li, Long Lu. Theoretical Study on the Mechanism of Continuous Preparation of 1,3,5-Triethory-2,4,6-Trinitrobenzene[J]. Acta Chimica Sinica, 2025, 83(4): 360-368.








| [1] |
Bottaro, J. C. Chem. Ind. 1996, 249.
|
| [2] |
Wang, W. J. J. Solid Rocket Technol. 2003, 26, 42. (in Chinese)
|
|
(王文俊, 固体火箭技术, 2003, 26, 42.)
|
|
| [3] |
Dong, H. S. Chin. J. Energ. Mater. 2004, A01, 1. (in Chinese)
|
|
(董海山, 含能材料, 2004, A01, 1.)
|
|
| [4] |
Sikder, A. K.; Sikder, N. J. Hazard. Mater. 2004, 112, 1.
pmid: 15225926 |
| [5] |
Huang, H.; Wang, Z. S.; Huang, H. J.; Li, J. S. Chin. J. Explos. Propellants 2005, 28, 9. (in Chinese)
|
|
(黄辉, 王泽山, 黄亨建, 李金山, 火炸药学报, 2005, 28, 9.)
|
|
| [6] |
Zhang, J. G.; Qin, J.; Klapötke, T. M. Chemistry of High Energy Materials, Beijing Institute of Technology Press, Beijing, 2016. (in Chinese)
|
|
(张建国, 秦涧译,Thomas M Klapötke, 高能材料化学, 北京理工大学出版社, 北京, 2016.)
|
|
| [7] |
Trache, D.; Klapötke, T. M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L. T. Green Chem. 2017, 19, 4711.
|
| [8] |
Huang, H. J.; Huang, H. Mater. China 2018, 37, 889. (in Chinese)
|
|
(黄亨建, 黄辉, 中国材料进展, 2018, 37, 889.)
|
|
| [9] |
Rice, S. F.; Simpson, R. L. Lawrence Livermore National Laboratory, Technical Report UCRL-LR-103683, 1990.
|
| [10] |
Dobratz, B. M. Los Alamos National Laboratory, Technical Report LA-13014-H, 1995.
|
| [11] |
Hou, C. H. Research on TATB-based Blunt Sensitive Explosive Transfer Agents, The Publishing House of Ordnance Industry, Beijing, 2021. (in Chinese)
|
|
(侯聪花, TATB基钝感传爆药研究, 兵器工业出版社, 北京, 2021.)
|
|
| [12] |
Jackson, C. J.; Wing, J. F. Ameri. Chem. J. 1887, 23, 138.
|
| [13] |
Moore, J. S.; Morrison, K. D.; Burnham, A. K.; Racoveanu, A.; Reynolds, J. G.; Koroglu, B.; Coffee, K. R. Propellants, Explos., Pyrotech. 2024, 49, e202400014
|
| [14] |
Bellamy, A. J.; Ward, S. J.; Golding, P. A. Propellants, Explos., Pyrotech. 2002, 27, 49.
|
| [15] |
Wang, H. C.; Wang, X. J.; Liu, X. X. Initiators Pyrotech. 2006, 5, 30. (in Chinese)
|
|
(王焕春, 王煊军, 刘祥萱, 火工品, 2006, 5, 30.)
|
|
| [16] |
Boddu, V. M.; Viswanath, D. S.; Ghosh, T. K.; Damavarapu, R. J. Hazard. Mater. 2010, 181, 1.
|
| [17] |
Straessler, N. A.; Velarde, S. P.US 2010/0317894 Al, 2010.
|
| [18] |
Yang, X. B. M.S. Thesis, Beijing Institute of Technology, Beijing, 2016
|
|
(杨学斌, 硕士论文, 北京理工大学, 北京, 2016.)
|
|
| [19] |
Huang, Y.M.S. Thesis, Nanjing University of Science & Technology, Nanjing, 2020
|
|
(黄瑶, 硕士论文, 南京理工大学, 南京, 2020.)
|
|
| [20] |
Lu, L.; Wu, Y. M.CN 117466741 A, 2023. (in Chinese)
|
|
(吕龙, 吴亚明,CN 117466741 A, 2023.)
|
|
| [21] |
Chakraborty, P.; Roy, S. C. Green Sustainable Chem. 2013, 3, 26.
|
| [22] |
Salami, S. A.; Siwe-Noundou, X.; Krause, R. W. Molecules 2022, 27, 4213.
|
| [23] |
See supporting information.
|
| [24] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, 386, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J.,Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.
|
| [25] |
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
|
| [26] |
Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215.
|
| [27] |
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
|
| [28] |
Fukui, K. Acc. Chem. Res. 1981, 14, 363.
|
| [1] | 崔勇康, 成守飞, 凌琳, 李玉学, 吕龙. 二氟氨基二硝甲基芳香杂环含能材料的理论研究[J]. 化学学报, 2024, 82(4): 377-386. |
| [2] | 母伟花, 马瑶, 方德彩, 王蓉, 张海娜. 1-碘-2-锂-邻碳硼烷与环戊二烯衍生物的类Diels-Alder反应的理论研究[J]. 化学学报, 2018, 76(1): 55-61. |
| [3] | 孟祥军, 郭小松, 贾俊芳, 和芹, 王一波. 5水合甘氨酸复合体的结构和性能的理论研究[J]. 化学学报, 2011, 69(01): 25-36. |
| [4] | 殷明,舒远杰,雄鹰,罗世凯,龙新平,朱祖良. 硝基咪唑化合物结构与性质的理论研究[J]. 化学学报, 2008, 66(19): 2117-2123. |
| [5] | 周中军 刘慧玲 黄旭日 孙家锺. 预测[Si, C, S]+和[Si, C, S]-体系的稳定异构体[J]. 化学学报, 2008, 66(14): 1637-1640. |
| [6] | 林宪杰, 徐为人, 武剑, 刘成卜. 苯甲醛肟与炔丙醇偶极环加成反应的理论研究[J]. 化学学报, 2007, 65(10): 930-936. |
| [7] | 孙政,曾小庆,王炜罡,葛茂发,王殿勋. 几种多氮杂环高能化合物的光电子能谱与紫外吸收光谱[J]. 化学学报, 2006, 64(3): 218-222. |
| [8] | 何文娣,周歌,胡海荣,田双河,田安民,文忠,赵鹏骥,徐起磊. 含能材料2,6-二胺-3,5-二硝基吡嗪-1-氧化物的B3LYP 研究[J]. 化学学报, 2001, 59(8): 1210-1215. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||