化学学报 ›› 2025, Vol. 83 ›› Issue (6): 551-556.DOI: 10.6023/A25040121 上一篇 下一篇
研究通讯
投稿日期:2025-04-16
发布日期:2025-05-13
基金资助:
Jian Lina, Hengyuan Lib, Bo Zhoub, Longwu Ye*( )
)
Received:2025-04-16
Published:2025-05-13
Contact:
*E-mail: longwuye@xmu.edu.cn; Tel.: 0592-2185833
Supported by:文章分享
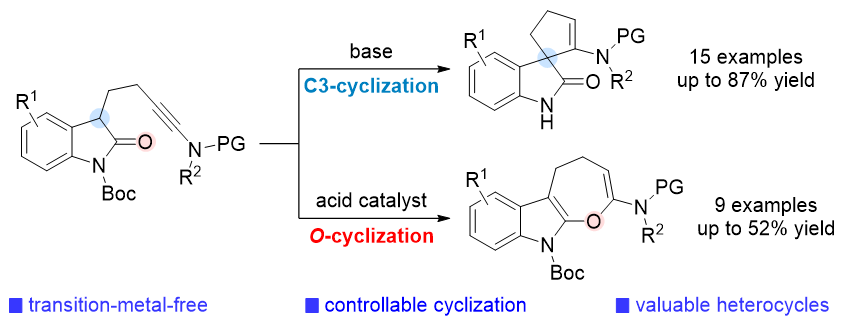
本工作报道了一例无过渡金属参与的炔酰胺与吲哚酮的多样性环化反应, 实现了螺环吲哚酮和吲哚并七元环骨架的高效合成. 通过反应条件的调节, 成功实现了位点可控的环化过程. 在碱促进的条件下, 发生2-吲哚酮C3位点的螺环化反应, 得到螺环吲哚酮产物. 在酸催化条件下, 通过2-吲哚酮的氧位点的选择性环化反应, 合成吲哚并七元环类化合物. 该类选择性环化反应具有催化剂及反应试剂廉价易得、操作简单、反应条件温和、底物适用范围良好等优势. 这项研究为具有潜在用途的螺环吲哚酮和吲哚并七元环类化合物的构筑提供了实用的新途径.
林健, 李恒渊, 周波, 叶龙武. 无过渡金属参与的炔酰胺与吲哚酮的多样性环化反应[J]. 化学学报, 2025, 83(6): 551-556.
Jian Lin, Hengyuan Li, Bo Zhou, Longwu Ye. Transition-Metal-Free Divergent Cyclization of Ynamides with Indolones[J]. Acta Chimica Sinica, 2025, 83(6): 551-556.
| Entry | Base | Conditions | Yieldb/% |
|---|---|---|---|
| 1 | Et3N | MeOH, 60 ℃, 14 h | <5 |
| 2 | DBU | MeOH, 60 ℃, 14 h | <5 |
| 3 | NaH | MeOH, 60 ℃, 6 h | 53 |
| 4 | KOH | MeOH, 60 ℃, 6 h | 59 |
| 5 | K3PO4 | MeOH, 60 ℃, 6 h | 65 |
| 6 | Na2CO3 | MeOH, 60 ℃, 6 h | 40 |
| 7 | Cs2CO3 | MeOH, 60 ℃, 6 h | 45 |
| 8 | K2CO3 | MeOH, 60 ℃, 6 h | 67 |
| 9 | K2CO3 | DCE, 60 ℃, 6 h | <5 |
| 10 | K2CO3 | THF, 60 ℃, 6 h | <5 |
| 11 | K2CO3 | PhMe, 60 ℃, 6 h | <5 |
| 12 | K2CO3 | DMF, 60 ℃, 6 h | 39 |
| 13 | K2CO3 | MeOH, 50 ℃, 6 h | 53 |
| 14c | K2CO3 | MeOH, 60 ℃, 6 h | 82 |
| Entry | Base | Conditions | Yieldb/% |
|---|---|---|---|
| 1 | Et3N | MeOH, 60 ℃, 14 h | <5 |
| 2 | DBU | MeOH, 60 ℃, 14 h | <5 |
| 3 | NaH | MeOH, 60 ℃, 6 h | 53 |
| 4 | KOH | MeOH, 60 ℃, 6 h | 59 |
| 5 | K3PO4 | MeOH, 60 ℃, 6 h | 65 |
| 6 | Na2CO3 | MeOH, 60 ℃, 6 h | 40 |
| 7 | Cs2CO3 | MeOH, 60 ℃, 6 h | 45 |
| 8 | K2CO3 | MeOH, 60 ℃, 6 h | 67 |
| 9 | K2CO3 | DCE, 60 ℃, 6 h | <5 |
| 10 | K2CO3 | THF, 60 ℃, 6 h | <5 |
| 11 | K2CO3 | PhMe, 60 ℃, 6 h | <5 |
| 12 | K2CO3 | DMF, 60 ℃, 6 h | 39 |
| 13 | K2CO3 | MeOH, 50 ℃, 6 h | 53 |
| 14c | K2CO3 | MeOH, 60 ℃, 6 h | 82 |
| Entry | Catalyst | Conditions | Yieldb/% | |
|---|---|---|---|---|
| 3a | 3a' | |||
| 1 | MsOH | DCE, 70 ℃, 6 h | <5 | 37 |
| 2 | TsOH | DCE, 70 ℃, 6 h | <5 | 43 |
| 3 | HNTf2 | DCE, 70 ℃, 0.5 h | <5 | 77 |
| 4 | TfOH | DCE, 70 ℃, 1 h | <5 | 84 |
| 5 | A1 | DCE, 70 ℃, 8 h | <5 | 65 |
| 6 | A2 | DCE, 70 ℃, 8 h | <5 | 68 |
| 7 | A3 | DCE, 70 ℃, 6 h | 30 | 12 |
| 8c | A3 | DCE, 70 ℃, 6 h | 48 | 9 |
| Entry | Catalyst | Conditions | Yieldb/% | |
|---|---|---|---|---|
| 3a | 3a' | |||
| 1 | MsOH | DCE, 70 ℃, 6 h | <5 | 37 |
| 2 | TsOH | DCE, 70 ℃, 6 h | <5 | 43 |
| 3 | HNTf2 | DCE, 70 ℃, 0.5 h | <5 | 77 |
| 4 | TfOH | DCE, 70 ℃, 1 h | <5 | 84 |
| 5 | A1 | DCE, 70 ℃, 8 h | <5 | 65 |
| 6 | A2 | DCE, 70 ℃, 8 h | <5 | 68 |
| 7 | A3 | DCE, 70 ℃, 6 h | 30 | 12 |
| 8c | A3 | DCE, 70 ℃, 6 h | 48 | 9 |
| [1] |
(a)
pmid: 18598018 |
|
(b)
pmid: 18598018 |
|
|
(c)
pmid: 18598018 |
|
|
(d)
pmid: 18598018 |
|
|
(e)
pmid: 18598018 |
|
|
(f)
doi: 10.1021/jm800194k pmid: 18598018 |
|
| [2] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [3] |
For selected examples on ring expansion, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [4] |
For selected examples on cycloaddition, see: (a)
pmid: 31318546 |
|
(b)
pmid: 31318546 |
|
|
(c)
pmid: 31318546 |
|
|
(d)
pmid: 31318546 |
|
|
(e)
doi: 10.1021/acs.joc.9b01268 pmid: 31318546 |
|
|
(f)
pmid: 31318546 |
|
| [5] |
(a)
pmid: 28688273 |
|
(b)
pmid: 28688273 |
|
|
(c)
pmid: 28688273 |
|
|
(d)
pmid: 28688273 |
|
|
(e)
doi: S0223-5234(17)30514-7 pmid: 28688273 |
|
| [6] |
(a)
pmid: 19736987 |
|
(b)
pmid: 19736987 |
|
|
(c)
pmid: 19736987 |
|
|
(d)
pmid: 19736987 |
|
|
(e)
pmid: 19736987 |
|
|
(f)
pmid: 19736987 |
|
|
(g)
doi: 10.1021/acs.joc.6b03090 pmid: 19736987 |
|
|
(h)
doi: 10.1021/acs.orglett.7b03030 pmid: 19736987 |
|
|
(i)
pmid: 19736987 |
|
|
(j)
doi: 10.1021/ja905302f pmid: 19736987 |
|
| [7] |
For selected examples on C3-addition, see: (a)
pmid: 28573864 |
|
(b)
pmid: 28573864 |
|
|
(c)
doi: 10.1021/acs.orglett.7b01233 pmid: 28573864 |
|
|
(d)
pmid: 28573864 |
|
|
(谢明胜, 武晓霞, 王刚, 林丽丽, 冯小明, 化学学报, 2014, 72, 856 ) .
doi: 10.6023/A13121253 pmid: 28573864 |
|
| [8] |
For selected examples on O-addition, see: (a)
|
|
(b)
|
|
| [9] |
For selected reviews on ynamide chemistry, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
| [10] |
For recent selected examples on transition-metal catalysis, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
|
(i)
|
|
|
(j)
|
|
|
(k)
|
|
|
(l)
|
|
|
(m)
|
|
|
(n)
|
|
|
(o)
|
|
|
For the related review, see: (p)
|
|
|
(q)
|
|
|
(贾世琨, 梅光建, 有机化学, 2023, 43, 2261 ) .
doi: 10.6023/cjoc202300035 |
|
|
(r)
|
|
|
(熊阳, 张国柱, 有机化学, 2023, 43, 1890 ) .
doi: 10.6023/cjoc202300028 |
|
| [11] |
For recent selected examples on organocatalysis, see: (a)
pmid: 31324800 |
|
(b)
pmid: 31324800 |
|
|
(翟彤仪, 葛畅, 钱鹏程, 周波, 叶龙武, 化学学报, 2023, 81, 1101 ) .
doi: 10.6023/A23040188 pmid: 31324800 |
|
|
(c)
pmid: 31324800 |
|
|
(d)
pmid: 31324800 |
|
|
(e)
pmid: 31324800 |
|
|
(f)
pmid: 31324800 |
|
|
(g)
pmid: 31324800 |
|
|
(h)
doi: 10.1038/s41467-019-11245-2 pmid: 31324800 |
| [1] | 李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚. Ni/Zn/Al类水滑石非贵金属催化剂的制备及其在醇胺法合成N-取代吲哚类化合物中的应用[J]. 化学学报, 2024, 82(7): 748-754. |
| [2] | 翟彤仪, 葛畅, 钱鹏程, 周波, 叶龙武. Brønsted酸催化炔酰胺分子内氢烷氧化/Claisen重排反应★[J]. 化学学报, 2023, 81(9): 1101-1107. |
| [3] | 王瑞祥, 赵庆如, 顾庆, 游书力. 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023, 81(5): 431-434. |
| [4] | 李速家, 吕健, 罗三中. 铟(I)/手性磷酸催化简单烯烃与β,γ-不饱和α-酮酸酯的不对称[4+2]环加成反应[J]. 化学学报, 2018, 76(11): 869-873. |
| [5] | 谭芬, 陈加荣, 王萍, 肖文精. Sc(OTf)3/Bis(oxazoline)复合物催化的2-芳基-1,3-茚二酮与2-乙烯基吲哚的不对称Diels-Alder反应:高效构建四氢咔唑螺茚酮衍生物[J]. 化学学报, 2014, 72(7): 836-840. |
| [6] | 李博, 周锐, 何谷, 郭丽, 黄维. 螺环吲哚类MDM2抑制剂的分子对接、定量构效关系和分子动力学模拟[J]. 化学学报, 2013, 71(10): 1396-1403. |
| [7] | 邱晃, 张丹, 刘顺英, 邱林, 周俊, 钱宇, 翟昌伟, 胡文浩. 通过对映选择性质子化实现吲哚的不对称C—H官能团化反应研究[J]. 化学学报, 2012, 70(24): 2484-2488. |
| [8] | 刘良军, 张国亮, 黄涛, 王玲, 孟琴. 酸性Fe-SiO2催化剂制备及其photo-Fenton催化性能[J]. 化学学报, 2011, 69(04): 452-458. |
| [9] | 张朝欣,达世俊,张化冰,孙彬,李瀛. 雌舞毒蛾引诱剂: (+)-(7R,8S)-7,8-环氧-2-甲基十八烷的不对称全合成[J]. 化学学报, 2007, 65(21): 2433-2436. |
| [10] | 廖菊芳, 邬泉周, 尹强, 王崇太, 李红玉, 李玉光. 磺酸功能化三维有序大孔材料的制备与催化性能研究[J]. 化学学报, 2006, 64(24): 2419-2424. |
| [11] | 姚瑞平, 张铭金, 杨俊,易德莲,徐君, 邓风,岳勇, 叶朝辉. SO3/γ-Al2O3固体酸催化剂的制备、结构与酸性表征[J]. 化学学报, 2005, 63(4): 269-273. |
| [12] | 孙渝,乐英红,高滋. 磷钨杂多酸及其铯盐上的常温正戊烷异构化反应[J]. 化学学报, 1998, 56(8): 792-798. |
| [13] | 吴毓敏,徐金锁,唐颐,高滋. 氧化锆层柱磷酸锆的制备及性能[J]. 化学学报, 1998, 56(11): 1099-1105. |
| [14] | 田来进,傅芳信,朱东升,潘华德,邢彦,林永华. β-烷氧羰基乙基三氯化锡配合物的合成、结构表征及酯交换反应[J]. 化学学报, 1997, 55(9): 884-891. |
| [15] | 高滋,唐颐. Y沸石的酸性[J]. 化学学报, 1990, 48(7): 632-638. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||