化学学报 ›› 2023, Vol. 81 ›› Issue (5): 431-434.DOI: 10.6023/A23030103 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究通讯
王瑞祥a, 赵庆如b, 顾庆b,*( ), 游书力a,b,*(
), 游书力a,b,*( )
)
投稿日期:2023-03-30
发布日期:2023-05-08
作者简介:基金资助:
Rui-Xiang Wanga, Qing-Ru Zhaob, Qing Gub( ), Shu-Li Youa,b(
), Shu-Li Youa,b( )
)
Received:2023-03-30
Published:2023-05-08
Contact:
*E-mail: qinggu@sioc.ac.cn; slyou@sioc.ac.cn
About author:Supported by:文章分享
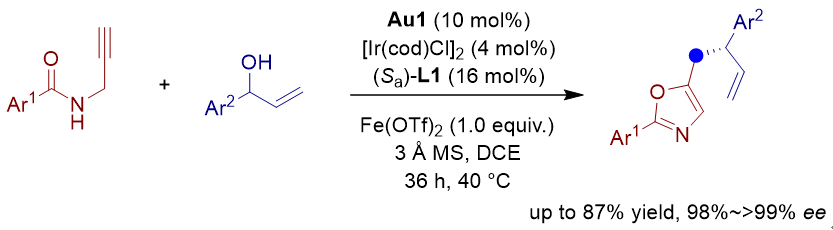
苄基亲核试剂参与的不对称烯丙基取代反应可以快速构筑含苄基片段的手性分子, 受到了有机化学家的广泛关注. 其中使用5-亚甲基二氢噁唑作为苄基亲核试剂等同体, 可以实现形式上的不对称苄位烯丙基取代反应. 然而由于5-亚甲基二氢噁唑化合物稳定性较差, 合成也存在一定困难, 对于发展高效的不对称苄位烯丙基取代反应提出了新挑战. 本工作发展了金/铱催化的炔基酰胺环化/不对称烯丙基苄基化串联反应. 首先金催化炔基酰胺环化生成5-亚甲基二氢噁唑, 随后亲核进攻烯丙基铱中间体, 以良好的收率(49%~87%)以及优秀的对映选择性控制(98%~>99% ee)得到芳基苄位烯丙基取代的手性分子.
王瑞祥, 赵庆如, 顾庆, 游书力. 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023, 81(5): 431-434.
Rui-Xiang Wang, Qing-Ru Zhao, Qing Gu, Shu-Li You. Gold/Iridium Catalyzed Alkynylamide Cyclization/Asymmetric Allylic Benzylation Cascade Reaction★[J]. Acta Chimica Sinica, 2023, 81(5): 431-434.
| Entry | [Au] (x mol%) | L | Solvent | Yield of 3ab | ee of 3ac |
|---|---|---|---|---|---|
| 1e | Au1 | (Sa)-L1 | DCE | 60% | >99% |
| 2 | Au1 | (Sa)-L1 | DCE | 95% (87%d) | >99% |
| 3f | Au1 | (Sa)-L1 | DCE | 94% | >99% |
| 4 | AuCl3 | (Sa)-L1 | DCE | 68% | >99% |
| 5 | Ph3PAuCl | (Sa)-L1 | DCE | 50% | >99% |
| 6 | Me2SAuCl | (Sa)-L1 | DCE | 75% | 49% |
| 7g | Ph3PAuMe | (Sa)-L1 | DCE | trace | N.D. h |
| 8 | Au1 | (Sa)-L2 | DCE | 36% | 52% |
| 9 | Au1 | (Sa)-L3 | DCE | 66% | 96% |
| 10 | Au1 | (Sa)-L1 | dioxane | 54% | >99% |
| 11 | Au1 | (Sa)-L1 | THF | 20% | N.D. h |
| 12 | Au1 | (Sa)-L1 | toluene | 52% | >99% |
| 13 | Au1 | (Sa)-L1 | MeCN | trace | N.D. h |
| 14 | Au1 | (Sa)-L1 | CHCl3 | 70% | >99% |
| Entry | [Au] (x mol%) | L | Solvent | Yield of 3ab | ee of 3ac |
|---|---|---|---|---|---|
| 1e | Au1 | (Sa)-L1 | DCE | 60% | >99% |
| 2 | Au1 | (Sa)-L1 | DCE | 95% (87%d) | >99% |
| 3f | Au1 | (Sa)-L1 | DCE | 94% | >99% |
| 4 | AuCl3 | (Sa)-L1 | DCE | 68% | >99% |
| 5 | Ph3PAuCl | (Sa)-L1 | DCE | 50% | >99% |
| 6 | Me2SAuCl | (Sa)-L1 | DCE | 75% | 49% |
| 7g | Ph3PAuMe | (Sa)-L1 | DCE | trace | N.D. h |
| 8 | Au1 | (Sa)-L2 | DCE | 36% | 52% |
| 9 | Au1 | (Sa)-L3 | DCE | 66% | 96% |
| 10 | Au1 | (Sa)-L1 | dioxane | 54% | >99% |
| 11 | Au1 | (Sa)-L1 | THF | 20% | N.D. h |
| 12 | Au1 | (Sa)-L1 | toluene | 52% | >99% |
| 13 | Au1 | (Sa)-L1 | MeCN | trace | N.D. h |
| 14 | Au1 | (Sa)-L1 | CHCl3 | 70% | >99% |
| [1] |
(a) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
doi: 10.1021/cr020027w |
|
(b) Lu, Z.; Ma, S. Angew. Chem. Int. Ed. 2008, 47, 258.
|
|
|
(c) Weaver, J. D.; Recio, A., III; Grenning, A. J.; Tunge, J. A. Chem. Rev. 2011, 111, 1846.
doi: 10.1021/cr1002744 |
|
|
For a book, see:
|
|
|
(d) Transition Metal Catalyzed Enantioselective Allylic Substitution: Organic Synthesis, Ed.: Kazmaier, U., Springer,eidelberg, 2012.
|
|
| [2] |
(a) Heterocycles in Natural Product Synthesis, Eds.: Majumdar, K. C.; Chattopadhyay, S. K., Wiley-VCH,Weinheim, 2011.
pmid: 25255204 |
|
(b) Metalation of Azoles and Related Five-Membered Ring Heterocycles: Topics in Heterocyclic Chemistry, Vol. 29, Ed.: Gribble, G. W., Springer, Heidelberg, 2012.
pmid: 25255204 |
|
|
(c) Heteroocyclic Chemistry in Drug Discovery, Ed.: Li, J.-J., Wiley, Hoboken, 2013.
pmid: 25255204 |
|
|
(d) Roughley, S. D.; Jordan, A. M. J. Med. Chem. 2011, 54, 3451.
doi: 10.1021/jm200187y pmid: 25255204 |
|
|
(e) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b pmid: 25255204 |
|
| [3] |
(a) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2008, 130, 14092.
doi: 10.1021/ja806781u |
|
(b) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2009, 131, 12056.
doi: 10.1021/ja904441a |
|
|
(c) Trost, B. M.; Thaisrivongs, D. A.; Hartwig, J. J. Am. Chem. Soc. 2011, 133, 12439.
doi: 10.1021/ja205523e |
|
| [4] |
(a) Sha, S.-C.; Jiang, H.; Mao, J.; Bellomo, A.; Jeong, S. A.; Walsh, P. J. Angew. Chem. Int. Ed. 2016, 55, 1070.
doi: 10.1002/anie.v55.3 |
|
(b) Mao, J.; Zhang, J.; Jiang, H.; Bellomo, A.; Zhang, M.; Gao, Z.; Dreher, S. D.; Walsh, P. J. Angew. Chem. Int. Ed. 2016, 55, 2526.
doi: 10.1002/anie.v55.7 |
|
| [5] |
(a) Mosrin, M.; Knochel, P. Org. Lett. 2009, 11, 1837.
doi: 10.1021/ol900342a pmid: 19317432 |
|
(b) Duez, S.; Steib, A. K.; Manolikakes, S. M.; Knochel, P. Angew. Chem. Int. Ed. 2011, 50, 7686.
doi: 10.1002/anie.201103074 pmid: 19317432 |
|
|
(c) Haas, D.; Hammann, J. M.; Greiner, R.; Knochel, P. ACS Catal. 2016, 6, 1540.
doi: 10.1021/acscatal.5b02718 pmid: 19317432 |
|
| [6] |
(a) Liu, X.-J.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 4002.
doi: 10.1002/anie.201700433 |
|
(b) Liu, X.-J.; Zhang, W.-Y.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, e202200164.
|
|
| [7] |
For selected reviews on Ir-catalyzed asymmetric allylic substitution reactions, see: a Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461.
pmid: 28649462 |
|
(b) Liu, W.-B.; Xia, J.-B.; You, S.-L. Top. Organomet. Chem. 2012, 38, 155.
pmid: 28649462 |
|
|
(c) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558.
doi: 10.1021/ar500167f pmid: 28649462 |
|
|
(d) Hethcox, J. C.; Shockley, S. E.; Stoltz, B. M. ACS Catal. 2016, 6, 6207.
doi: 10.1021/acscatal.6b01886 pmid: 28649462 |
|
|
(e) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539.
doi: 10.1021/acs.accounts.7b00300 pmid: 28649462 |
|
|
(f) Rössler, S. L.; Petrone, D. A.; Carreira, E. M. Acc. Chem. Res. 2019, 52, 2657.
doi: 10.1021/acs.accounts.9b00209 pmid: 28649462 |
|
|
(g) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855.
doi: 10.1021/acs.chemrev.8b00506 pmid: 28649462 |
|
| [8] |
(a) Singha, S.; Serrano, E.; Mondal, S.; Daniliuc, C. G.; Glorius, F. Nat. Catal. 2020, 3, 48.
doi: 10.1038/s41929-019-0387-3 pmid: 35476460 |
|
(b) Butcher, T. W.; Yang, J. L.; Amberg, W. M.; Watkins, N. B.; Wilkinson, N. D.; Hartwig, J. F. Nature 2020, 583, 548.
doi: 10.1038/s41586-020-2399-1 pmid: 35476460 |
|
|
(c) Chen, P.; Li, Y.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Angew. Chem. Int. Ed. 2020, 59, 7083.
doi: 10.1002/anie.v59.18 pmid: 35476460 |
|
|
(d) Han, M.; Yang, M.; Wu, R.; Li, Y.; Jia, T.; Gao, Y.; Ni, H.-L.; Hu, P.; Wang, B.-Q.; Cao, P. J. Am. Chem. Soc. 2020, 142, 13398.
doi: 10.1021/jacs.0c01766 pmid: 35476460 |
|
|
(e) Tu, H.-F.; Yang, P.; Lin, Z.; Zheng, C.; You, S.-L. Nat. Chem. 2020, 12, 838.
doi: 10.1038/s41557-020-0489-1 pmid: 35476460 |
|
|
(f) Rossi-Ashton, J. A.; Clarke, A. K.; Donald, J. R.; Zheng, C.; Taylor, R. J. K.; Unsworth, W. P.; You, S.-L. Angew. Chem. Int. Ed. 2020, 59, 7598.
doi: 10.1002/anie.202001956 pmid: 35476460 |
|
|
(g) Wang, J.; Qi, X.; Min, X.-L.; Yi, W.; Liu, P.; He, Y. J. Am. Chem. Soc. 2021, 143, 10686.
doi: 10.1021/jacs.1c04400 pmid: 35476460 |
|
|
(h) Davis, C. R.; Luvaga, I. K.; Ready, J. M.; J. Am. Chem. Soc. 2021, 143, 4921.
doi: 10.1021/jacs.1c01242 pmid: 35476460 |
|
|
(i) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380.
doi: 10.1126/science.abd6095 pmid: 35476460 |
|
|
(j) Crisenza, G. E. M.; Faraone, A.; Gandolfo, E.; Mazzarella, D.; Melchiorre, P. Nat. Chem. 2021, 13, 575.
doi: 10.1038/s41557-021-00683-5 pmid: 35476460 |
|
|
(k) Xiao, L.; Wei, L.; Wang, C.-J. Angew. Chem. Int. Ed. 2021, 60, 24930.
doi: 10.1002/anie.v60.47 pmid: 35476460 |
|
|
(l) Peng, Y.; Huo, X.; Luo, Y.; Wu, L.; Zhang, W. Angew. Chem. Int. Ed. 2021, 60, 24941.
doi: 10.1002/anie.v60.47 pmid: 35476460 |
|
|
(m) Zhang, M.-M.; Chen, P.; Xiong, W.; Hui, X.-S.; Lu, L.-Q.; Xiao, W.-J. CCS Chem. 2021, 3, 3383.
pmid: 35476460 |
|
|
(n) Deng, Y.; Liang, X.; Wei, K.; Yang, Y.-R. J. Am. Chem. Soc. 2021, 143, 20622.
doi: 10.1021/jacs.1c11265 pmid: 35476460 |
|
|
(o) Zhao, Q.-R.; Jiang, R.; You, S.-L. Acta Chim. Sinica 2021, 79, 1107. (in Chinese)
doi: 10.6023/A21070320 pmid: 35476460 |
|
|
(赵庆如, 蒋茹, 游书力, 化学学报, 2021, 79, 1107.)
doi: 10.6023/A21070320 pmid: 35476460 |
|
|
(p) Yang, P.-S.; Liu, C.-X.; Zhang, W.-W.; You, S.-L. Acta Chim. Sinica 2021, 79, 742. (in Chinese)
doi: 10.6023/A21050198 pmid: 35476460 |
|
|
(杨普苏, 刘晨旭, 张文文, 游书力, 化学学报, 2021, 79, 742.)
doi: 10.6023/A21050198 pmid: 35476460 |
|
|
(q) Ding, L.; Song, H.; Zheng, C.; You, S.-L. J. Am. Chem. Soc. 2022, 144, 4770.
doi: 10.1021/jacs.2c01103 pmid: 35476460 |
|
|
(r) Liu, X.-J.; Zhang, W.-Y.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, 61, e2022001.
pmid: 35476460 |
|
|
(s) Moghadam, F. A.; Hicks, E. F.; Sercel, Z. P.; Cusumano, A. Q.; Bartberger, M. D.; Stoltz, B. M. J. Am. Chem. Soc. 2022, 144, 7983.
doi: 10.1021/jacs.2c02960 pmid: 35476460 |
|
|
(t) Yang, P.; Wang, R.-X.; Cheng, Y.-Z.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, 61, e202213520.
pmid: 35476460 |
|
|
(u) Yang, W.-L.; Shang, X.-Y.; Luo, X.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202203661.
pmid: 35476460 |
|
|
(v) Jiang, R.; Zhao, Q.-R.; Zheng, C.; You, S.-L. Nat. Catal. 2022, 5, 1089.
doi: 10.1038/s41929-022-00879-z pmid: 35476460 |
|
|
(w) Zhang, J.; Yang, W.-L.; Zheng, H.; Wang, Y.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202117079.
pmid: 35476460 |
|
|
(x) Wu, Z.-H.; Wang, H.-Y.; Yang, H.-L.; Wei, L.-H.; Hayashi, T.; Duan, W.-L. Angew. Chem. Int. Ed. 2022, 61, e202213904.
pmid: 35476460 |
|
|
(y) Yang, W.-L.; Shang, X.-Y.; Ni, T.; Yan, H.; Luo, X.; Zhen, H.; Li, Z.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202210207.
pmid: 35476460 |
|
|
(z) Fu, C.; Xiong, Q.; Xiao, L.; He, L.; Bai, T.; Zhang, Z.; Dong, X.-Q.; Wang, C.-J. Chin. J. Chem. 2022, 40, 1059.
doi: 10.1002/cjoc.v40.9 pmid: 35476460 |
|
|
(aa) Tang, X.; Su, Z.; Lin, Q.; Lin, L.; Dong, S.; Feng, X. Chin. J. Chem. 2022, 40, 1793.
doi: 10.1002/cjoc.v40.15 pmid: 35476460 |
|
|
(ab) Jia, S.-H.; Chen, S.-Y.; Liu, Z.-S.; Cheng, H.-G.; Zhou, Q.-H. Chin. J. Org. Chem. 2022, 42, 3373. (in Chinese)
doi: 10.6023/cjoc202209002 pmid: 35476460 |
|
|
(贾仕虎, 陈思元, 刘泽水, 程鸿刚, 周强辉, 有机化学, 2022, 42, 3373.)
doi: 10.6023/cjoc202209002 pmid: 35476460 |
|
|
(ac) Xie, J.-H.; Hou, Y.-M.; Feng, Z.; You, S.-L. Angew. Chem. Int. Ed. 2023, 63, e202216396.
pmid: 35476460 |
|
| [9] |
Liu, X.-J.; Zheng, C.; Yang, Y.-H.; Jin, S.; You, S.-L. Angew. Chem. Int. Ed. 2019, 58, 10493.
doi: 10.1002/anie.v58.31 |
| [10] |
(a) Seppanen, O.; Aikonen, S.; Muuronen, M.; Alamillo-Ferrer, C.; Bures, J.; Helaja, J. Chem. Commun. 2020, 56, 14697.
doi: 10.1039/D0CC05999D |
|
(b) Yang, G.; Ke, Y.-M.; Zhao., Y. Angew. Chem. Int. Ed. 2021, 60, 12775.
doi: 10.1002/anie.v60.23 |
|
|
(c) Ma, Y.; Ali, H. S.; Hussein, A. A. Catal. Sci. Technol. 2022, 12, 674.
doi: 10.1039/D1CY01617B |
|
| [11] |
(a) Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S. Chem. Rev. 2020, 120, 13382.
doi: 10.1021/acs.chemrev.0c00245 |
|
(b) Wei, L.; Wang, C.-J. Chem Catal. 2023, 3, 100455.
|
| [1] | 郭姿珠, 张睿, 孙旦, 王海燕, 黄小兵, 唐有根. 无负极锂金属电池在局部高浓度电解液中的产气研究[J]. 化学学报, 2024, 82(9): 919-924. |
| [2] | 热孜古丽•玉努斯, 卡迪尔亚•阿布都外力, 罗时玮, 阿布都热西提•阿布力克木. 电化学条件下由二苄胺合成N-亚苄基苄胺及衍生化研究[J]. 化学学报, 2024, 82(8): 843-848. |
| [3] | 苑志祥, 张雅岚, 张浩, 张仕杰, 王朵, 张波涛, 张建军, 崔光磊. 高杨氏模量细菌纤维素隔膜有效抑制锂枝晶[J]. 化学学报, 2024, 82(8): 849-855. |
| [4] | 罗倩钰, 汪诚艳, 张天龙, 夏培元, 张潇, 杨明. 金属离子-吉西他滨单磷酸酯络合物纳米粒用于胰腺癌治疗的研究[J]. 化学学报, 2024, 82(7): 772-781. |
| [5] | 王程鋆, 王悦靓, 王会巧, 邓兆祥. 金-银纳米异质组装过程的化学不相容性[J]. 化学学报, 2024, 82(7): 763-771. |
| [6] | 李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚. Ni/Zn/Al类水滑石非贵金属催化剂的制备及其在醇胺法合成N-取代吲哚类化合物中的应用[J]. 化学学报, 2024, 82(7): 748-754. |
| [7] | 王筑城, 刘磊, 朱梦媛, 孙悦, 赵晴, 丁玉寅, 陆继鑫, 王存国, 李奇, 贺爱华, 叶付臣. 1,5-二氨基蒽醌(AAQ)复合材料用作锂离子电池新型正极材料的性能研究[J]. 化学学报, 2024, 82(6): 589-595. |
| [8] | 郑灏宁, 刘金宇. 有机催化吲哚碳环官能团化研究进展[J]. 化学学报, 2024, 82(6): 641-657. |
| [9] | 张仕杰, 王朵, 崔浩然, 张雅岚, 张浩, 苑志祥, 韩鹏献, 姚树玉, 黄浪, 张建军, 崔光磊. 聚环氧乙烷固态聚合物电解质基室温固态锂金属电池的研究进展[J]. 化学学报, 2024, 82(6): 690-706. |
| [10] | 王南南, 陈玉贞. CoNi-MOF-74/泡沫镍衍生的CoNi@C/NF复合物用于高效有机电合成[J]. 化学学报, 2024, 82(6): 621-628. |
| [11] | 周何鑫, 崔青云, 胡雪敏, 杨文秀, 田肖, 王硕. 金属有机框架衍生氮掺杂碳限域钴原子簇催化硝基化合物转移加氢[J]. 化学学报, 2024, 82(5): 503-510. |
| [12] | 陈元金, 黄大江, 石向辉, 席振峰, 魏俊年. 还原条件下非对称钳形PNN钴配合物的反应性研究[J]. 化学学报, 2024, 82(5): 471-476. |
| [13] | 谷琪, 刘夏夏, 周鑫宇, 李江, 林秀婧, 马延文. 用于锂金属电池的聚合物固态电解质的研究进展[J]. 化学学报, 2024, 82(4): 449-457. |
| [14] | 王敏, 陈帮塘, 陈桥林, 王俊, 陈名钊, 蒋志龙, 王平山. 6,6″-二(2,6-二甲氧基苯)-三联吡啶及其衍生物金属配位自组装的研究进展[J]. 化学学报, 2024, 82(3): 336-347. |
| [15] | 刘洋, 高丰琴, 马占营, 张引莉, 李午戊, 侯磊, 张小娟, 王尧宇. 一例钴基金属有机框架化合物活化过氧单硫酸盐高效降解水中亚甲基蓝研究[J]. 化学学报, 2024, 82(2): 152-159. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||