化学学报 ›› 2011, Vol. 69 ›› Issue (17): 1965-1972. 上一篇 下一篇
研究论文
孙廷利1,王玉东1,张晨曦1,孙孝敏1,2,胡敬田*,1
Sun Tingli1 Wang Yudong1 Zhang Chenxi1 Sun Xiaomin1,2 Hu Jingtian*,1
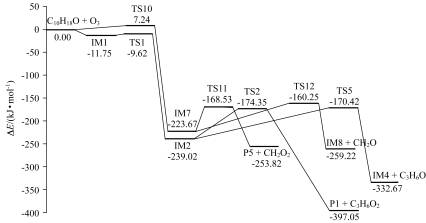
采用密度泛函理论研究了大气中芳樟醇臭氧化反应机理. 在B3LYP/6-31G*水平上对该反应体系的反应物、中间体、过渡态及产物进行了几何构型优化和频率计算, 在B3LYP/6-311+G(3df,2pd)水平下进行单点能量计算, 构筑了反应的势能剖面. 对反应中间体与H2O和NO可能的氧化机理进行了详细的描述. 并对转化过程中各竞争反应的反应势垒、反应热和Gibbs自由能等物理化学参数进行了详细分析. 生成的产物极性和水溶性增强, 易通过成核、水合或吸附反应形成二次有机气溶胶.