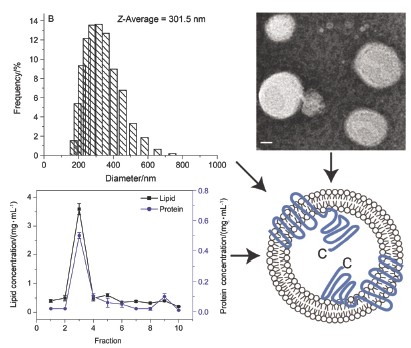
| [1] Lowy, F. D. N. Engl. J. Med. 1998, 339, 520. [2] Emori, T. G.; Gaynes, R. P. Clin. Microbiol. Rev. 1993, 6, 428.[3] Klevens, R. M.; Morrison, M. A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L. H.; Lynfield, R.; Dumyati, G.; Townes, J. M.; Craig, A. S.; Zell, E. R.; Fosheim, G. E.; McDougal, L. K.; Carey, R. B.; Fridkin, S. K. JAMA. 2007, 298, 1763. [4] De Lencastre, H.; Oliveira, D.; Tomasz, A. Curr. Opin. Microbiol. 2007, 10, 428. [5] Novick, R. P. Mol. Microbiol. 2003, 48, 1429.[6] Stauff, D. L.; Skaar, E. P. Contrib. Microbiol. 2009, 16, 120. [7] Cheung, A. L.; Nishina, K. A.; Trotonda, M. P.; Tamber, S. Int. J. Biochem. Cell. Biol. 2008, 40, 355. [8] Novick, R. P.; Ross, H. F.; Projan, S. J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. EMBO J. 1993, 12, 3967. [9] Thoendel, M.; Kavanaugh, J. S.; Flack, C. E.; Horswill, A. R. Chem. Rev. 2011, 111, 117. [10] Geisinger, E.; George, E. A.; Muir, T. W.; Novick, R. P. J. Biol. Chem. 2008, 283, 8930. [11] Geisinger, E.; Muir, T. W.; Novick, R. P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 1216. [12] Grebe, T. W.; Stock, J. B. Adv. Microb. Physiol. 1999, 41, 139.[13] George Cisar, E. A.; Geisinger, E.; Muir, T. W.; Novick, R. P. Mol. Microbiol. 2009, 74, 44. [14] Taubes, G. Science. 2008, 321, 356. [15] Chambers, H. F.; Deleo F. R. Nat. Rev. Microbiol. 2009, 7, 629. [16] Gordon, C. P.; Williams, P.; Chan, W. C. J. Med. Chem. 2013, 56, 1389. [17] Gotoh, Y.; Eguchi, Y.; Watanabe, T.; Okamoto, S.; Doi, A.; Utsumi, R. Curr. Opin. Microbiol. 2010, 13, 232. [18] Sanowar, S.; LeMoual, H. Biochem. J. 2005, 390, 769. [19] Martin, M.; Albanesi, D.; Alzari, P. M.; de Mendoza, D. Protein Expr. Purif. 2009, 66, 39. [20] Janausch, I. G.; Garcia-Moreno, I.; Unden, G. J. Biol. Chem. 2002, 277, 39809. [21] Liu, C. G.; Quan, C. S.; Wang, J. F.; Yu, G. M.; Fan, S. D. China Biotechnology 2012, 32, 1. (刘春光, 权春善, 王剑锋, 于桂梅, 范圣第, 中国生物工程杂志, 2012, 32, 1.)[22] Wang, L. N.; Quan, C. S.; Liu, B. Q.; Wang, J. F.; Xiong, W.; Zhao, P. C.; Fan, S. D. PLoS ONE. 2013, 8, e80400. doi: 10. 1371/journal. pone. 0080400. [23] Lina, G.; Jarraud, S.; Ji, G.; Greenland, T.; Pedraza, A.; Etienne, J.; Novick, R. P.; Vandenesch, F. Mol. Microbiol. 1998, 28, 655. [24] Hofmann, K.; Stoffel, W. Biol. Chem. Hoppe-Seyler 1993, 347, 166. [25] Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E. L. J. Mol. Biol. 2001, 305, 567. [26] Li, W.; Wang, J. H.; Quan, C. S.; Zheng, W.; Fan, S. D. Acta Biophysica Sinica 2009, 25, 219. (李巍, 王军华, 权春善, 郑维, 范圣第, 生物物理学报, 2009, 25, 219.)[27] Claros, M. G.; von Heijne, G. Comput. Appl. Biosci. 1994, 10, 685. [28] Bernsel, A.; Viklund, H.; Hennerdal, A.; Elofsson, A. Nucleic Acids Res. 2009, 37, W465. [29] Hirokawa, T.; Boon-Chieng, S.; Mitaku, S. Bioinformatics 1998, 14, 378. [30] Jones, D. T.; Taylor, W. R.; Thornton, J. M. Biochemistry 1994, 33, 3038. [31] McGuffin, L. J.; Bryson, K.; Jones, D. T. Bioinformatics 2000, 16, 404. [32] Wang, L. N.; Quan, C. S.; Xiong, W.; Qu, X. J.; Fan, S. D. BBA-Biomembranes 2013, (Available online 17 December 2013. DOI information: 10. 1016/j. bbamem. 2013. 12. 006). [33] Van Den Ent, F.; Löwe, J. J. Biochem. Biophys. Methods 2006, 67, 67. [34] Wang, L. N.; Quan, C. S.; Liu, B. Q.; Xu, Y. B.; Zhao, P. C.; Xiong, W.; Fan, S. D. Int. J. Mol. Sci. 2013, 14, 18470. [35] Rigaud, J. L.; Lévy, D. Methods Enzymol. 2003, 372, 65. [36] Bartlett, G. R. J. Biol. Chem. 1959, 234, 466. [37] Quan, C. S.; Ma, J. L.; Wang, X.; Li, W.; Fan, S. D. Acta Chim. Sinica 2010, 68, 1167. (权春善, 马金龙, 王雪, 李巍, 范圣第, 化学学报, 2010, 68, 1167.)[38] Sanowar, S.; LeMoual, H. Biochem. J. 2005, 390, 769. |