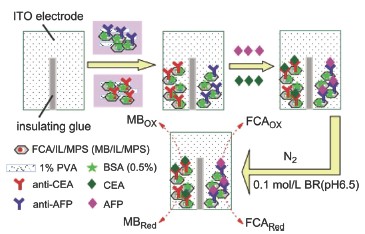
| [1] Wu, J.; Fu, Z. F.; Yan, F.; Ju, H. X. Trends Anal. Chem. 2007, 26, 679.[2] Wilson, M. S.; Nie, W. Y. Anal. Chem. 2006, 78, 6476.[3] Wu, J.; Yan, Y. T.; Yan, F.; Ju, H. X. Anal. Chem. 2008, 80(15), 6072.[4] Yang, Z. J.; Liu, H.; Zong, C.; Yan, F.; Ju, H. X. Anal. Chem. 2009, 81, 5484.[5] Tlili, C.; Myunga, N. V.; Shettyb, V.; Mulchandani, A. Biosens. Bioelectron. 2011, 26, 4382.[6] Luo, Y.; Chen, M.; Wen, Q. J.; Zhao, M.; Zhang, B.; Li, X. Y.; Wang, F.; Huang, Q.; Yao, C. Y.; Jiang, T. L.; Cai, J. R.; Fu, W. L. Clin. Chem. 2006, 52, 2273.[7] Lee, H. J.; Namkoong, K.; Cho, E. C.; Ko, C.; Park, J. C.; Lee, S. S. Biosens. Bioelectron. 2009, 24, 3120.[8] Zhang, J.; Lang, H. P.; Huber, F.; Bietsch, A.; Grange, W.; Certa, U.; Mckendry, R.; Güntherodt, H. J.; Hegner, M.; Gerber, C. Nat. Nanotechnol. 2006, 1, 214.[9] Nasef, H.; Beni, V.; Ozalp, V. C.; O'Sullivan, C. K. Anal. Bioanal. Chem. 2010, 396, 2565.[10] Loyprasert, S.; Thavarungkui, P.; Asawatreatanakul, P.; Wongkittisuksa, B.; Limsakul, C.; Kanatharana, P. Biosens. Bioelectron. 2008, 24, 78.[11] Zhang, D. D.; Peng, Y. G.; Qi, H. G.; Gao, Q.; Zhang, C. X. Biosens. Bioelectron. 2010, 25, 1088.[12] Escosura-Muñiz, A. D. L.; Merkoçi, A. Electrochem. Commun. 2010, 12, 859.[13] Bayley, H.; Cremer, P. S. Nature 2001, 413, 226.[14] Martin, C. R.; Siwy, Z. Nat. Mater. 2004, 3, 284.[15] Lin, J. H.; Wei, Z. J.; Chu, P. F. Anal. Biochem. 2012, 421, 97.[16] Lin, J. H.; Wei, Z. J.; Zhang, H. H.; Shao, M. J. Biosens. Bioelectron. 2013, 41, 342.[17] Lin, J. H.; Wei, Z. J.; Mao, C. M. Biosens. Bioelectron. 2011, 29, 40.[18] Zhao, D. Y.; Huo, Q. S.; Feng, J. L.; Chmelka, B. F.; Stucky, G. D. J. Am. Chem. Soc. 1998, 120, 6024.[19] Zhao, D. Y.; Feng, J. L.; Huo, Q. S.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D. Science 1998, 279, 548.[20] Lin, J. H.; Zhang, H. H.; Chu, P. F. Acta Chim. Sinica 2012, 70, 2372. (林洁华, 张慧慧, 楚鹏飞, 化学学报, 2012, 70, 2372.)[21] Sun, J. M.; Ma, D.; Zhang, H.; Liu, X. M.; Han, X. W.; Bao, X. H.; Weinberg, G.; Pfander, N.; Su, D. S. J. Am. Chem. Soc. 2006, 128, 15756.[22] Buzzeo, M. C.; Evans, R. G.; Compton, R. G. ChemPhysChem 2004, 5, 1106.[23] Endres, F. Zeitschrift Fur Physikalische Chemie. 2004, 218, 255.[24] Lin, J. H.; Qu, W.; Zhang, S. S. Anal. Biochem. 2007, 360, 288. |