化学学报 ›› 2019, Vol. 77 ›› Issue (9): 906-910.DOI: 10.6023/A19020070 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
林凤闺蓉a, 梁宇杰a, 郦鑫耀a, 宋颂a*, 焦宁ab*( )
)
投稿日期:2019-02-27
发布日期:2019-03-22
通讯作者:
宋颂,焦宁
E-mail:ssong@bjmu.edu.cn;jiaoning@pku.edu.cn
基金资助:
Lin, Fengguironga, Liang, Yujiea, Li, Xinyaoa, Song, Songa*, Jiao, Ningab*( )
)
Received:2019-02-27
Published:2019-03-22
Contact:
Song, Song,Jiao, Ning
E-mail:ssong@bjmu.edu.cn;jiaoning@pku.edu.cn
Supported by:文章分享
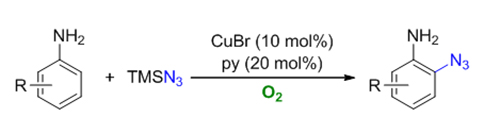
叠氮取代的苯胺是有机合成中应用广泛的结构单元. 通过C—H键活化策略来制备叠氮基苯胺衍生物往往需要使用当量的剧烈氧化剂, 造成反应的整体原子经济性低, 官能团耐受性差. 本文使用廉价、绿色的氧气作为最终氧化剂, 发展了高效的铜催化苯胺C—H键叠氮化的方法. 该转化具有反应条件温和、区域选择性单一和官能团兼容性广等优点.
林凤闺蓉, 梁宇杰, 郦鑫耀, 宋颂, 焦宁. 氧气氧化铜催化的苯胺邻位叠氮化反应[J]. 化学学报, 2019, 77(9): 906-910.
Lin, Fengguirong, Liang, Yujie, Li, Xinyao, Song, Song, Jiao, Ning. Copper-catalyzed ortho C-H Azidation of Anilines Using Molecular Oxygen as Terminal Oxidant[J]. Acta Chimica Sinica, 2019, 77(9): 906-910.
| [1] | (a) Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V . Angew. Chem. Int. Ed. 2005, 44, 5188. |
| (b) Iha, R. K.; Wooley, K. L.; Nyström, A. M.; Burke, D. J.; Kade, M. J.; Hawker, C. J . Chem. Rev. 2009, 109, 5620 | |
| (c) Lallana, E.; Riguera, R.; Fernandez‐Megia, E . Angew. Chem. Int. Ed. 2011, 50, 8794. | |
| (d) Schilling, C. I.; Jung, N.; Biskup, M.; Schepers, U.; Brase, S . Chem. Soc. Rev. 2011, 40, 4840. | |
| (e) Li, G.; Bai, R . Prog. Chem. 2011, 23, 1692 (in Chinese). | |
| ( 李光, 白如科 , 化学进展, 2011, 23, 1692.) | |
| (f) Zhang, W.; Xu, W.; Kuang, C . Chin. J. Org. Chem. 2015, 35, 2059 (in Chinese). | |
| ( 张文生, 许文静, 匡春香 , 有机化学, 2015, 35, 2059.) | |
| (g) Wang, Y.; Liu, L.; Wang, Y . Chin. J. Org. Chem. 2018, 38, 1009 (in Chinese). | |
| ( 王钰莹, 刘莉, 王也铭 , 有机化学, 2018, 38, 1009.) | |
| (h) Yan, J.; Ji, X.; Hua, S.; Wang, J . Chin. J. Org. Chem. 2018, 38, 791 (in Chinese). | |
| ( 严珺, 姬晓悦, 花书贵, 王静 , 有机化学, 2018, 38, 791.) | |
| (i) Huang, Q.; Tan, Z.; Yu, C.; Zhu, X.; Wu, L . Chin. J. Org. Chem. 2017, 37, 97 (in Chinese). | |
| ( 黄青兰, 谭志强, 于长江, 朱晓敏, 吴禄勇 , 有机化学, 2017, 37, 97.) | |
| [2] |
For some selected examples in recent years: (a) Goswami, M.; de Bruin, B . Eur. J. Org. Chem. 2017,1152
doi: 10.6023/cjoc201206023 |
|
(b) Lapointe, G.; Kapat, A.; Weidner, K.; Renaud, P . Pure Appl. Chem. 2012, 84, 1633
doi: 10.6023/cjoc201206023 |
|
|
(c) Wu, K.; Liang, Y.; Jiao, N . Molecules. 2016, 21, 352.
doi: 10.6023/cjoc201206023 |
|
|
(d) Jiang, Y.; Kuang, C.; Han, C.; Wang, H.; Liang, X . Chin. J. Org. Chem. 2012, 32, 2231 (in Chinese).
doi: 10.6023/cjoc201206023 |
|
|
( 江玉波, 匡春香, 韩春美, 王红, 梁雪秋 , 有机化学, 2012, 32, 2231.)
doi: 10.6023/cjoc201206023 |
|
|
(e) Ritchie, C. D.; Wright, D. J . J. Am. Chem. Soc. 1971, 93, 2429.
doi: 10.6023/cjoc201206023 |
|
|
(f) Ollivier, C.; Renaud, P . J. Am. Chem. Soc. 2000, 122, 6496.
doi: 10.6023/cjoc201206023 |
|
|
(g) Zhu, W.; Ma, D . Chem. Commun. 2004, 888.
doi: 10.6023/cjoc201206023 |
|
|
(h) Banert, K.; Berndt, C.; Firdous, S.; Hagedorn, M.; Joo, Y.-H.; Rüffer, T.; Lang, H . Angew. Chem. Int. Ed. 2010, 49, 10206.
doi: 10.6023/cjoc201206023 |
|
|
(i) Kapat, A.; König, A.; Montermini, F.; Renaud, P . J. Am. Chem. Soc. 2011, 133, 13890.
doi: 10.6023/cjoc201206023 |
|
|
(j) Liu, C.; Wang, X.; Li, Z.; Cui, L.; Li, C . J. Am. Chem. Soc. 2015, 137, 9820.
doi: 10.6023/cjoc201206023 |
|
|
(k) Sun, X.; Li, X.; Song, S.; Zhu, Y.; Liang, Y.-F.; Jiao, N . J. Am. Chem. Soc. 2015, 137, 6059.
doi: 10.6023/cjoc201206023 |
|
|
(l) Song, S.; Feng, P.; Zou, M.; Jiao, N . Chin. J. Chem. 2017, 35, 845.
doi: 10.6023/cjoc201206023 |
|
|
(m) Cong, F.; Wei, Y.; Tang, P . Chem. Commun. 2018, 54, 4473.
doi: 10.6023/cjoc201206023 |
|
|
(n) Kong, L.; Hu, J.; Kong, L . J. Zhejiang Univ. (Nat. Sci.). 2010, 33, 185 (in Chinese).
doi: 10.6023/cjoc201206023 |
|
|
( 孔黎春, 胡洁玲, 孔龙峰 , 浙江师范大学学报(自然科学版), 2010, 33, 185.)
doi: 10.6023/cjoc201206023 |
|
|
(o) Yang, B.; Ren, X.; Shen, X.; Li, T.; Lu, Z . Chin. J. Chem. 2018, 36, 1017.
doi: 10.6023/cjoc201206023 |
|
|
(p) Peng, H.; Yuan, Z.; Chen, P.; Liu, G . Chin. J. Chem. 2017, 35, 876.
doi: 10.6023/cjoc201206023 |
|
|
(q) Yuan, Y.-A.; Lu, D.-F.; Chen, Y.-R.; Xu, H . Angew. Chem. Int. Ed. 2016, 55, 534.
doi: 10.6023/cjoc201206023 |
|
|
(r) Fu, N.; Sauer, G. S.; Saha, A.; Loo, A.; Lin, S . Science. 2017, 357, 575.
doi: 10.6023/cjoc201206023 |
|
|
(s) Shen, S.-J.; Zhu, C.-L.; Lu, D.-F.; Xu, H . ACS Catal. 2018, 8, 447.
doi: 10.6023/cjoc201206023 |
|
|
(t) Shen, K.; Wang, Q . J. Am. Chem. Soc. 2017, 139, 13110.
doi: 10.6023/cjoc201206023 |
|
|
(u) Siu, J. C.; Parry, J. B.; Lin, S . J. Am. Chem. Soc. 2019, 141, 2825.
doi: 10.6023/cjoc201206023 |
|
|
(v) Xu, L.; Chen, J.; Chu, L . Org. Chem. Front. 2019, 6, 512.
doi: 10.6023/cjoc201206023 |
|
|
(w) Wang, F.; Qi, X.; Liang, Z.; Chen, P.; Liu, G . Angew. Chem. Int. Ed. 2014, 53, 1881.
doi: 10.6023/cjoc201206023 |
|
|
(x) Wang, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G . Angew. Chem. Int. Ed. 2017, 56, 2054.
doi: 10.6023/cjoc201206023 |
|
|
(y) Wu, Z.; Ren, R.; Zhu, C . Angew. Chem. Int. Ed. 2016, 55, 10821.
doi: 10.6023/cjoc201206023 |
|
|
(z) Bunescu, A.; Ha, T. M.; Wang, Q.; Zhu, J . Angew. Chem. Int. Ed. 2017, 56, 10555.
doi: 10.6023/cjoc201206023 |
|
|
(aa) Bao, X.; Wang, Q.; Zhu, J . Nat. Commun. 2019, 10, 769.
doi: 10.6023/cjoc201206023 |
|
|
(ab) Zhou, P.; Lin, L.; Chen, L.; Zhong, X.; Liu, X.; Feng, X . J. Am. Chem. Soc. 2017, 139, 13414.
doi: 10.6023/cjoc201206023 |
|
|
(ac) Xie, Z.; Deng, J.; Qiu, Z.; Li, J.; Zhu, Q . Chem. Commun. 2016, 52, 6467.
doi: 10.6023/cjoc201206023 |
|
| [3] | (a) Huang, X.; Groves, J. T . ACS Catal. 2016, 6, 751. |
| (b) Vita, M. V.; Waser, J . Angew. Chem. Int. Ed. 2015, 54, 5290. | |
| (c) Shilov, A. E.; Shul'pin, G. B . Chem. Rev. 1997, 97, 2879. | |
| (d) Liang, Y.; Liang, Y.-F.; Jiao, N . Org. Chem. Front. 2015, 2, 403. | |
| (e) Magnus, P.; Lacour, J . J. Am. Chem. Soc. 1992, 114, 767. | |
| (f) Zhdankin, V. V.; Krasutsky, A. P.; Kuehl, C. J.; Simonsen, A. J.; Woodward, J. K.; Mismash, B.; Bolz, J. T . J. Am. Chem. Soc. 1996, 118, 5192. | |
| (g) Deng, Q.-H.; Bleith, T.; Wadepohl, H.; Gade, L. H . J. Am. Chem. Soc. 2013, 135, 5356. | |
| (h) Sharma, A.; Hartwig, J. F . Nature. 2015, 517, 600. | |
| (i) Huang, X.; Bergsten, T. M.; Groves, J. T . J. Am. Chem. Soc. 2015, 137, 5300. | |
| (j) Wang, Y.; Li, G.-X.; Yang, G.; He, G.; Chen, G . Chem. Sci. 2016, 7, 2679. | |
| (k) Zhang, X.; Yang, H.; Tang, P . Org. Lett. 2015, 54, 5828. | |
| (l) Vita, M. V.; Waser, J . Angew. Chem. Int. Ed. 2015, 54, 5290. | |
| (m) Rabet, P. T. G.; Fumagalli, G.; Boyd, S.; Greaney, M. F . Org. Lett. 2016, 18, 1646. | |
| (n) Li, X.; Shi, Z.-J . Org. Chem. Front. 2016, 3, 1326. | |
| (o) Hendrick, C. E.; Bitting, K. J.; Cho, S.; Wang, Q . J. Am. Chem. Soc. 2017, 139, 11622. | |
| (p) Dou, Y.; Ying, S.; Zhang, C.; Yu, L.; Zheng, K.; Zhu, Q . Prog. Chem. 2017, 29, 293 (in Chinese). | |
| ( 窦言东, 应莎莎, 张晨卿, 余黎阳, 郑垦, 朱勍 , 化学进展, 2017, 29, 293.) | |
| [4] | Grieb, P . Philos. Trans. R. Soc. Lond. 1864, 13, 377. |
| [5] |
(a) He, Z.; Bae, M.; Wu, J.; Jamison, T. F . Angew. Chem., Int. Ed. 2014, 53, 14451
doi: 10.1002/anie.201408522 |
|
(b) Xie, F.; Qi, Z.; Li, X . Angew. Chem. Int. Ed. 2013, 52, 11862.
doi: 10.1002/anie.201408522 |
|
|
(c) Dou, Y.; Xie, Z.; Sun, Z.; Fang, H.; Shen, C.; Zhang, P.; Zhu, Q . ChemCatChem 2016, 8, 3570.
doi: 10.1002/anie.201408522 |
|
|
(d) Hussain, M. K.; Ansari, M. I.; Kant, R.; Hajela, K . Org. Lett. 2014, 16, 560.
doi: 10.1002/anie.201408522 |
|
|
(e) Azad, C. S.; Narula, A. K . RSC Adv. 2015, 5, 100223.
doi: 10.1002/anie.201408522 |
|
|
(f) Yan, Y.-M.; Gao, Y.; Ding, M.-W . Tetrahedron. 2016, 72, 5548.
doi: 10.1002/anie.201408522 |
|
|
(g) Yamamoto, K.; Kamino, S.; Sawada, D . Tetrahedron Lett. 2017, 58, 3936.
doi: 10.1002/anie.201408522 |
|
|
(h) Dhineshkumar, J.; Gadde, K.; Prabhu, K. R . J. Org. Chem. 2018, 83, 228.
doi: 10.1002/anie.201408522 |
|
|
(i) Dou, Y.; Yin, B.; Zhang, P.; Zhu, Q . Eur. J. Org. Chem. 2018, 2018, 4571.
doi: 10.1002/anie.201408522 |
|
| [6] |
Tang, C.; Jiao, N . J. Am. Chem. Soc. 2012, 134, 18924.
doi: 10.1021/ja3089907 |
| [7] |
Fan, Y.; Wan, W.; Ma, G.; Gao, W.; Jiang, H.; Zhu, S.; Hao, J. Chem. Commun. 2014, 50, 5733.
doi: 10.1039/C4CC01481B |
| [8] |
Fang, H.; Dou, Y.; Ge, J.; Chhabra, M.; Sun, H.; Zhang, P.; Zheng, Y.; Zhu, Q . J. Org. Chem. 2017, 82, 11212.
doi: 10.1021/acs.joc.7b01594 |
| [9] |
(a) Liang, Y.-F.; Wu, K.; Liu, Z.; Wang, X.; Liang, Y.; Liu, C.; Jiao, N . Sci. China Chem. 2015, 58, 1334.
doi: 10.1007/s11426-015-5363-4 |
|
(b) Huang, X.; Li, X.; Zou, M.; Song, S.; Tang, C.; Yuan, Y.; Jiao, N . J. Am. Chem. Soc. 2014, 136, 14858
doi: 10.1007/s11426-015-5363-4 |
|
|
(c) Liang, Y.; Jiao, N . Angew. Chem. Int. Ed. 2016, 55, 4035.
doi: 10.1007/s11426-015-5363-4 |
|
|
(d) Shi, Z.; Zhang, C.; Li, S.; Pan, D.; Ding, S.; Cui, Y.; Jiao, N . Angew. Chem. Int. Ed. 2009, 48, 4572.
doi: 10.1007/s11426-015-5363-4 |
|
|
(e) Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N . Angew. Chem. Int. Ed. 2017, 56, 2487.
doi: 10.1007/s11426-015-5363-4 |
|
|
(f) Zhang, C.; Xu, Z.; Zhang, L.; Jiao, N . Angew. Chem. Int. Ed. 2011, 50, 11088.
doi: 10.1007/s11426-015-5363-4 |
|
|
(g) Li, X.; Jiao, N . Chin. J. Chem. 2017, 35, 1349.
doi: 10.1007/s11426-015-5363-4 |
|
| [10] | (a) Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Chem. Rev. 2005, 105, 2329 |
| (b) Stahl, S. S . Angew. Chem. Int. Ed. 2004, 43, 3400. | |
| (c) Sigman, M. S.; Jensen, D. R . Acc. Chem. Res. 2006, 39, 221. | |
| (d) Gligorich, K. M.; Sigman, M. S . Angew. Chem. Int. Ed. 2006, 45, 6612. | |
| (e) Wendlandt, A. E.; Suess, A. M.; Stahl, S. S . Angew. Chem. Int. Ed. 2011, 50, 11062. | |
| (f) Shi, Z.; Zhang, C.; Tang, C.; Jiao, N . Chem. Soc. Rev. 2012, 41, 3381. | |
| (g) Campbell, A. N.; Stahl, S. S . Acc. Chem. Res. 2012, 45, 851. | |
| (h) Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C . Chem. Rev. 2013, 113, 6234. | |
| (i) Serrano-Plana, J.; Garcia-Bosch, I.; Company, A.; Costas, M . Acc. Chem. Res. 2015, 48, 2397. | |
| (j) Li, X.; Jiao, N . Chin. J. Chem. 2017, 35, 1349. | |
| (k) Liang, Y.-F.; Jiao, N . Acc. Chem. Res. 2017, 50, 1640. | |
| (l) Wang, D.; Weinstein, A. B.; White, P. B.; Stahl, S. S . Chem. Rev. 2018, 118, 2636. | |
| (m) Wertz, S.; Studer, A . Green Chem. 2013, 15, 3116. | |
| (n) Parmeggiani, C.; Cardona, F . Green Chem. 2012, 14, 547. | |
| (o) Xu, H.; Tang, R.; Gong, N.; Liu, C.; Zhou, Y . Prog. Chem. 2007, 19, 1736 (in Chinese). | |
| ( 许海峰, 唐瑞仁, 龚年华, 刘长辉, 周亚平 , 化学进展, 2007, 19, 1736); | |
| (p) Wang, J.; Deng, W.; Wang, Y.; Liu, L.; Guo, Q . Chin. J. Org. Chem. 2006, 26, 397 (in Chinese). | |
| ( 王嘉瑞, 邓维, 王晔峰, 刘磊, 郭庆祥 , 有机化学, 2006, 26, 397). | |
| [11] |
(a) Zhang, C.; Jiao, N . Angew. Chem. Int. Ed. 2010, 49, 6174.
doi: 10.1002/anie.201001651 |
|
(b) Lu, W.; Xi, C. Tetrahedron Lett. 2008, 49, 4011.
doi: 10.1002/anie.201001651 |
|
|
(c) Wang, J.; He, J.; Zhi, C.; Luo, B.; Li, X.; Pan, Y.; Cao, X.; Gu, H . RSC Adv. 2014, 4, 16607.
doi: 10.1002/anie.201001651 |
|
| [12] |
(a) Corma, A.; Serna, P Science. 2006, 313, 332
doi: 10.6023/cjoc201607030 |
|
(b) Zhou, P.; Jiang, L.; Wang, F.; Deng, K.; Lv, K.; Zhang, Z. Sci. Adv. 2017, 3, 1601945;
doi: 10.6023/cjoc201607030 |
|
|
(c) Zhang, N.; Xu, Y.-J . Chem. Mater. 2013, 25, 1979.
doi: 10.6023/cjoc201607030 |
|
|
(d) Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J . Appl. Catal., B 2018, 227, 386;
doi: 10.6023/cjoc201607030 |
|
|
(e) Füldner, S.; Mild, R.; Siegmund, H. I.; Schroeder, J. A.; Gruber, M.; König, B . Green Chem. 2010, 12, 400.
doi: 10.6023/cjoc201607030 |
|
|
(f) Kadam, H. K.; Tilve, S. G . RSC Adv. 2015, 5, 83391.
doi: 10.6023/cjoc201607030 |
|
|
(g) Li, Y.; Li, J.; Peng, L.; Gao, L.; Jin, K.; Sheng, L.; Zhang, N.; Wang, S.; Li, J . Chin. J. Org. Chem. 2017, 37, 485. (in Chinese).
doi: 10.6023/cjoc201607030 |
|
|
( 李英俊, 李继阳, 彭立娜, 高立信, 靳焜, 盛丽, 张楠, 王思远, 李佳, 有机化学, 2017, 37, 485).
doi: 10.6023/cjoc201607030 |
|
|
(h) Liu, J.; Lin, C.; Wan, Y.; Song, H . Chin. J. Org. Chem. 2008, 28, 317. (in Chinese).
doi: 10.6023/cjoc201607030 |
|
|
( 刘进兵, 林崇懒, 万一千, 宋化灿, 有机化学, 2008, 28, 317).
doi: 10.6023/cjoc201607030 |
|
| [13] |
Lin, W.; Zhang, X.; He, Z.; Jin, Y.; Gong, L.; Mi, A. Synth. Commun. 2002, 32, 3279.
doi: 10.1081/SCC-120014032 |
| [1] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [2] | 高杨, 张学鑫, 余金生, 周剑. α-手性三级叠氮化合物的不对称催化合成新进展★[J]. 化学学报, 2023, 81(11): 1590-1608. |
| [3] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [4] | 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63. |
| [5] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [6] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| [7] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| [8] | 张欣欣, 刘荣, 王蕾, 付宏刚. 细菌纤维素基柔性锌离子电池正极的构筑及性能研究[J]. 化学学报, 2021, 79(5): 670-677. |
| [9] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [10] | 罗潇, 焦宁. 三氟乙酸酐(TFAA)促进的Stieglitz重排反应制备苯胺[J]. 化学学报, 2020, 78(8): 758-762. |
| [11] | 杨晶亮, 杨伟民, 林嘉盛, 汪安, 徐娟, 李剑锋. 电场强度对等离激元诱导热电子的影响[J]. 化学学报, 2020, 78(7): 670-674. |
| [12] | 代迷迷, 王健, 李麟阁, 王琪, 刘美男, 张跃钢. 界面增强的CeO2/FeNi MOF高效析氧催化剂[J]. 化学学报, 2020, 78(4): 355-362. |
| [13] | 张荣华, 许冰, 张展鸣, 张俊良. Ming-Phos/铜催化的亚甲胺叶立德与硝基烯烃的不对称[3+2]环加成反应[J]. 化学学报, 2020, 78(3): 245-249. |
| [14] | 于越, 张新波. 多孔金属有机框架材料作为锂金属负极保护层助力长寿命锂氧气电池[J]. 化学学报, 2020, 78(12): 1434-1440. |
| [15] | 黄浩, 林华鑫, 王敏, 廖建. 1,2-苯基异噁唑为氮源的铜催化苯乙烯不对称硼胺化[J]. 化学学报, 2020, 78(11): 1229-1234. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||