化学学报 ›› 2021, Vol. 79 ›› Issue (8): 1073-1081.DOI: 10.6023/A21030083 上一篇 下一篇
研究论文
徐平a, 张西华a,*( ), 马恩a, 饶富a, 刘春伟b, 姚沛帆a, 孙峙b,*(
), 马恩a, 饶富a, 刘春伟b, 姚沛帆a, 孙峙b,*( ), 王景伟a
), 王景伟a
投稿日期:2021-03-07
发布日期:2021-07-19
通讯作者:
张西华, 孙峙
基金资助:
Ping Xua, Xihua Zhanga( ), En Maa, Fu Raoa, Chunwei Liub, Peifan Yaoa, Zhi Sunb(
), En Maa, Fu Raoa, Chunwei Liub, Peifan Yaoa, Zhi Sunb( ), Jingwei Wanga
), Jingwei Wanga
Received:2021-03-07
Published:2021-07-19
Contact:
Xihua Zhang, Zhi Sun
Supported by:文章分享

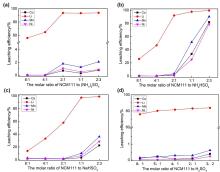
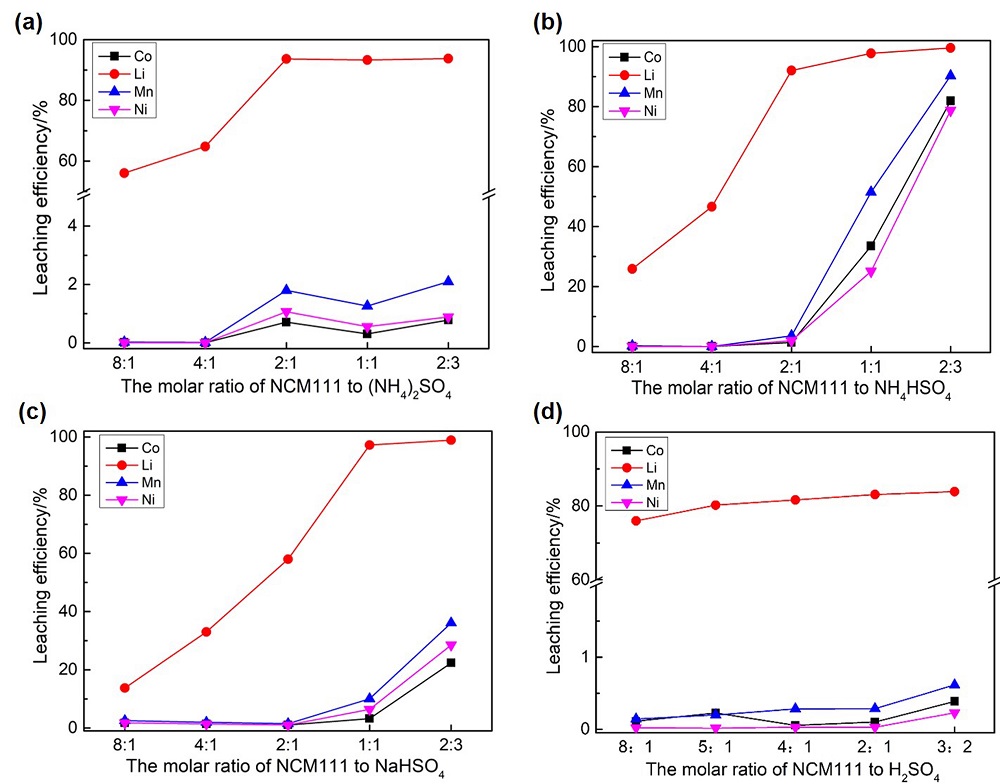
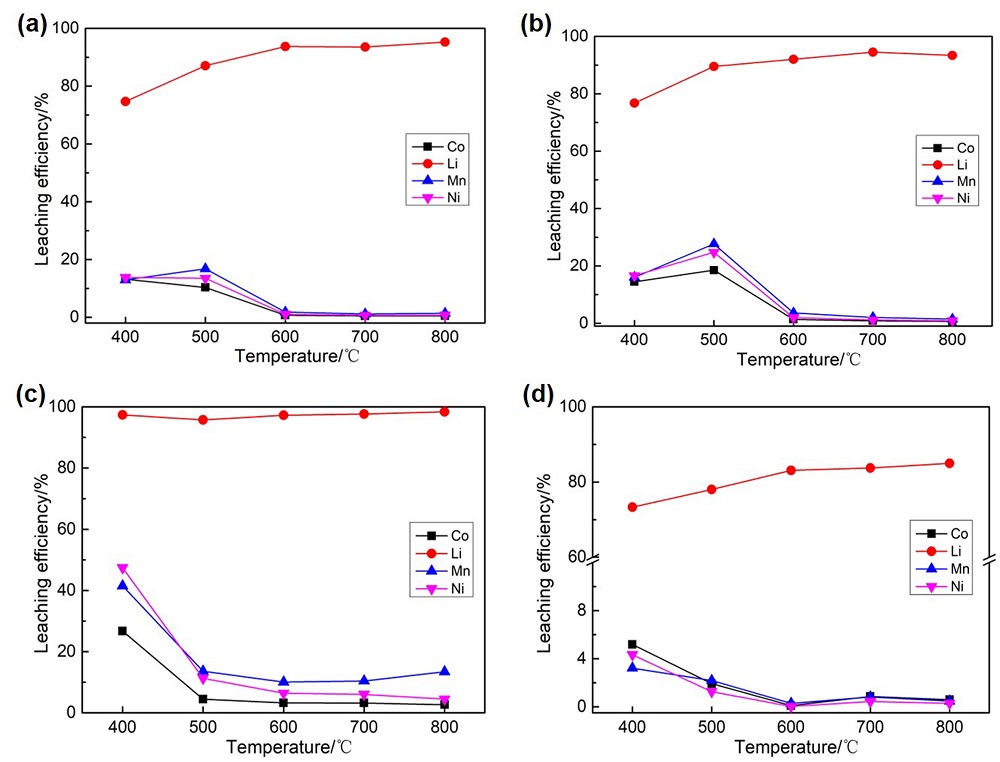
针对当前退役锂离子电池有价金属提取工艺选择性差、环境风险突出的瓶颈问题, 提出了碳/硫协同选择性提锂的新思路. 首先在系统考察(NH4)2SO4、NH4HSO4、NaHSO4和H2SO4分别作为焙烧剂对退役锂离子电池LiNi1/3Co1/3Mn1/3O2正极粉末中锂的浸出选择性、环境友好性和经济性影响的基础上, 确定H2SO4为最佳焙烧剂. 基于此, 研究了石墨添加量对LiNi1/3Co1/3Mn1/3O2中锂的浸出选择性的影响, 揭示了C/S协同强化锂的浸出选择性的转化路径及其机制. 结果表明, 在LiNi1/3Co1/3Mn1/3O2与H2SO4物质的量比为2∶1、石墨添加量为20% (w)、焙烧温度为600 ℃、焙烧时间为120 min的最优条件下, LiNi1/3Co1/3Mn1/3O2中锂的浸出率高达93%, 回收的Li2CO3纯度高于电池级Li2CO3纯度要求; Ni、Co和Mn均进入渣相, 经分离纯化后可作为合成正极材料的前驱物, 分离得到的石墨可回用于硫化焙烧过程的添加剂. 通过对LiNi1/3Co1/3Mn1/3O2混合粉末(含20 wt.%石墨)硫化焙烧热行为及其产物X射线衍射(XRD)表征表明, 石墨的添加降低了硫化焙烧的反应温度, 通过C/S协同作用强化了LiNi1/3Co1/3Mn1/3O2中锂的选择性浸出, 且不产生SOx等有毒有害气体. 本工作结合硫化焙烧和碳热还原优势, 为退役锂离子电池正负极材料的同步循环利用开辟了新思路, 实现了LiNi1/3Co1/3Mn1/3O2中锂的高效选择性清洁提取和废石墨负极的循环利用.
徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081.
Ping Xu, Xihua Zhang, En Ma, Fu Rao, Chunwei Liu, Peifan Yao, Zhi Sun, Jingwei Wang. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements[J]. Acta Chimica Sinica, 2021, 79(8): 1073-1081.
| Composition | Li | Ni | Co | Mn | Al | Fe |
|---|---|---|---|---|---|---|
| Content (w)/% | 7.78 | 22.67 | 18.28 | 16.21 | 0.34 | 0.04 |
| Composition | Li | Ni | Co | Mn | Al | Fe |
|---|---|---|---|---|---|---|
| Content (w)/% | 7.78 | 22.67 | 18.28 | 16.21 | 0.34 | 0.04 |
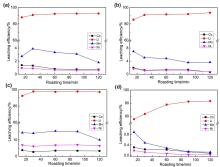
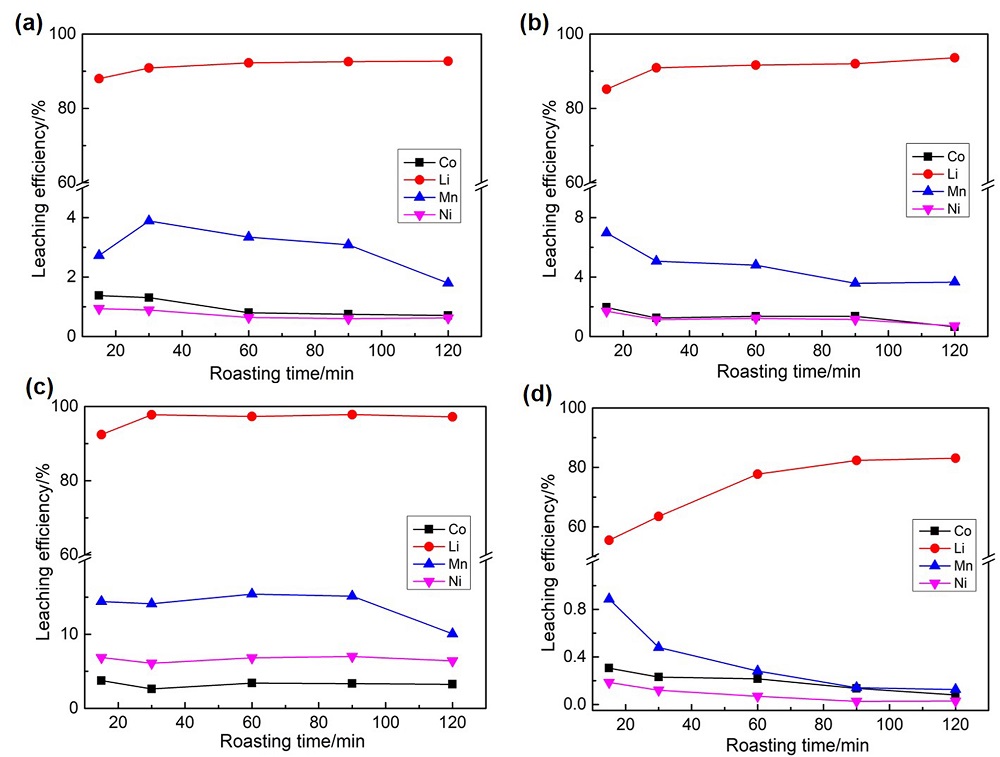
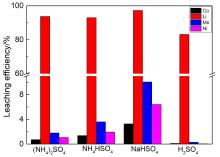
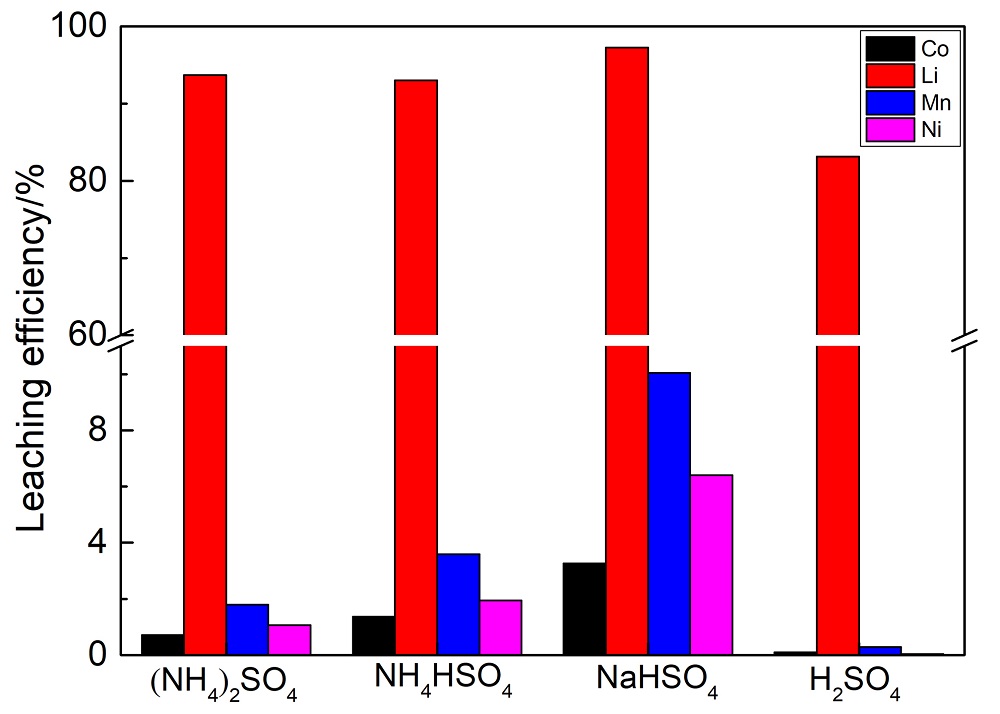
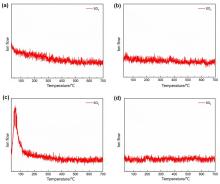
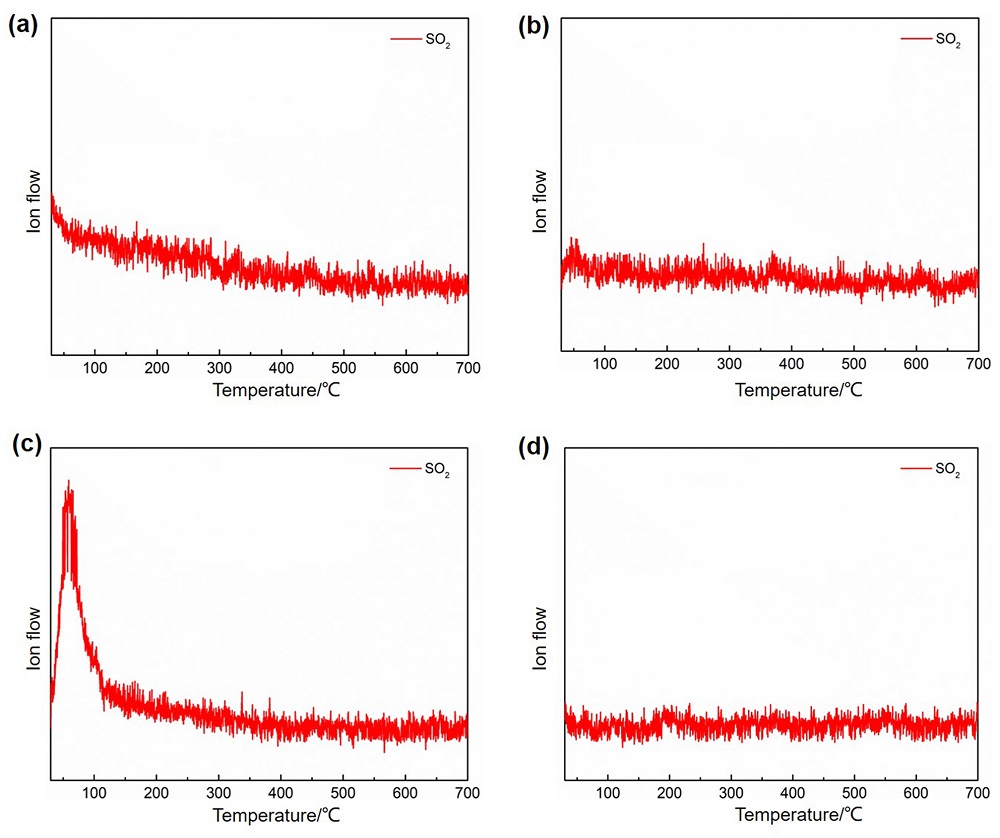
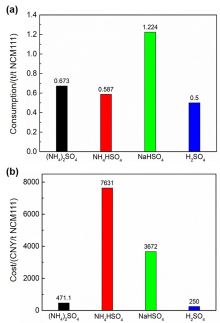

| Composition | Li | Ni | Co | Mn | Na | Others |
|---|---|---|---|---|---|---|
| Content (w)/% | 99.64 | 0.01 | 0.03 | 0.02 | 0.08 | 0.22 |
| Composition | Li | Ni | Co | Mn | Na | Others |
|---|---|---|---|---|---|---|
| Content (w)/% | 99.64 | 0.01 | 0.03 | 0.02 | 0.08 | 0.22 |

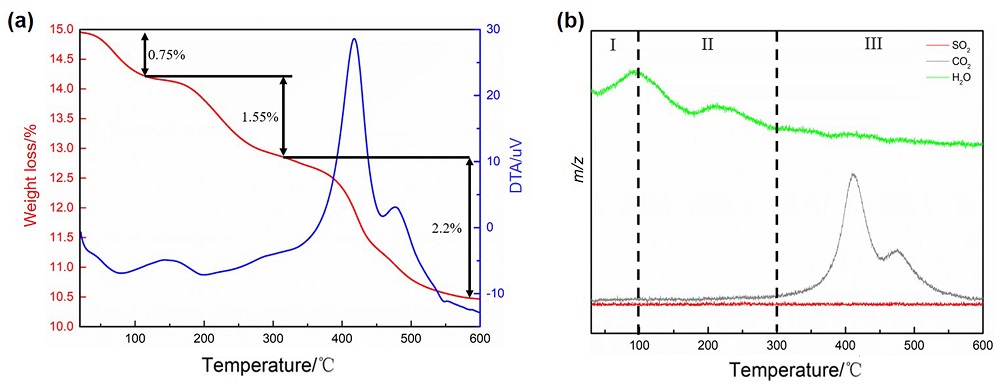
| [1] |
Zeng, X. L.; Li, J. H.; Singh, C. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129.
doi: 10.1080/10643389.2013.763578 |
| [2] |
Wang, M. M.; Zhang, C. C.; Zhang, F. S. Waste Manage. 2016, 51, 239.
doi: 10.1016/j.wasman.2016.03.006 |
| [3] |
Nie, H. H.; Xu, L.; Song, D. W.; Song, J. S.; Shi, X. X.; Wang, X. Q.; Zhang, L. Q.; Yuan, Z. H. Green Chem. 2015, 17, 1276.
doi: 10.1039/C4GC01951B |
| [4] |
Li, L.; Bian, Y. F.; Zhang, X. X.; Guan, Y. B.; Fan, E. S.; Wu, F.; Chen, R. J. Waste Manage. 2018, 71, 362.
doi: S0956-053X(17)30774-2 pmid: 29110940 |
| [5] |
Yang, Y.; Xu, S. M.; He, Y. H. Waste Manage. 2017, 64, 219.
doi: S0956-053X(17)30165-4 pmid: 28336333 |
| [6] |
Pagnanelli, F.; Moscardini, E.; Granata, G.; Cerbelli, S.; Agosta, L.; Fieramosca, A.; Toro, L. J. Ind. Eng. Chem. 2014, 20, 3201.
doi: 10.1016/j.jiec.2013.11.066 |
| [7] |
Mishra, D.; Kim, D. J.; Ralph, D.; Ahn, J. G.; Rhee, Y. H. Waste Manage. 2008, 28, 333.
doi: 10.1016/j.wasman.2007.01.010 |
| [8] |
Meshram, P.; Abhilash,
|
| [9] |
Kim, S.; Yang, D.; Rhee, K.; Sohn, J. Res. Chem. Intermed. 2014, 40, 2447.
doi: 10.1007/s11164-014-1653-2 |
| [10] |
Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; Abbott, A.; Ryder, K.; Gaines, L.; Anderson, P. Nature 2019, 575, 75.
doi: 10.1038/s41586-019-1682-5 |
| [11] |
Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. J. Power Sources 2012, 207, 173.
doi: 10.1016/j.jpowsour.2012.01.152 |
| [12] |
Dewulf, J.; Vorst, G. V.D.; Denturck, K.; Langenhove, H. V.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. J.R. Resour. Conserv. Recycl. 2010, 54, 229.
doi: 10.1016/j.resconrec.2009.08.004 |
| [13] |
Ma, Y. Y.; Tang, J. J.; Wanaldi, R.; Zhou, X. Y.; Wang, H.; Zhou, C. Y.; Yang, J. J. Hazard Mater. 2020, 123491.
|
| [14] |
Li, H.; Xing, S. Z.; Liu, Y.; Li, F. J.; Guo, H.; Kuang, G. ACS Sustainable Chem. Eng. 2017, 5, 8017.
doi: 10.1021/acssuschemeng.7b01594 |
| [15] |
He, L. P.; Sun, S. Y.; Song, X. F.; Yu, J. G. Waste Manage. 2017, 64, 171.
doi: 10.1016/j.wasman.2017.02.011 |
| [16] |
Xiao, J. F.; Li, J.; Xu, Z. M.J Environ. Sci. Technol. 2017, 51, 11960.
doi: 10.1021/acs.est.7b02561 |
| [17] |
Hu, J. T.; Zhang, J. L.; Li, H. X.; Chen, Y. Q.; Wang, C. Y. J. Power Sources 2017, 351, 192.
doi: 10.1016/j.jpowsour.2017.03.093 |
| [18] |
Wang, D. H.; Zhang, X. D.; Chen, H. J.; Sun, J. Y. Miner. Eng. 2018, 126, 28.
doi: 10.1016/j.mineng.2018.06.023 |
| [19] |
Wang, D. H.; Wen, H.; Chen, H. J.; Yang, Y. J.; Liang, H. Y.J. Chem. Res. Chin. Univ. 2016, 32, 674.
doi: 10.1007/s40242-016-5490-2 |
| [20] |
Peng, C.; Liu, F. P.; Wang, Z. L.; Wilson, B. P.; Lundström, M. J. Power Sources 2019, 415, 179.
doi: 10.1016/j.jpowsour.2019.01.072 |
| [21] |
Sun, J. Y.M.S. Thesis, Lanzhou University of Technology, Lanzhou, 2015 (in Chinese.)
|
|
( 孙建勇, 硕士论文, 兰州理工大学, 兰州, 2015.)
|
|
| [22] |
Wen, H. M.S. Thesis, Lanzhou University of Technology, Lanzhou, 2016 (in Chinese.)
|
|
( 文豪, 硕士论文, 兰州理工大学, 兰州, 2016.)
|
|
| [23] |
Lin, J.; Liu, C. W.; Cao, H. B.; Chen, R. J.; Yang, Y. X.; Li, L.; Sun, Z. Green Chem. 2019, 21, 5904.
doi: 10.1039/C9GC01350D |
| [24] |
Lin, J.; Li, L.; Fan, E. S.; Liu, C. W.; Zhang, X. D.; Cao, H. B.; Sun, Z.; Chen, R. J. ACS Appl. Mater. Interfaces 2020, 12, 18482.
doi: 10.1021/acsami.0c00420 |
| [25] |
Yang, Y. X.; Meng, X. Q.; Cao, H. B.; Lin, X.; Liu, C. W.; Sun, Y.; Zhang, Y.; Sun, Z. Green Chem. 2018, 20, 3121.
doi: 10.1039/C7GC03376A |
| [1] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [2] | 苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊. 离子聚合原位固态化构建高安全锂电池固态聚合物电解质的研究进展★[J]. 化学学报, 2023, 81(8): 1064-1080. |
| [3] | 李子奇, 刘力玮, 毛承晖, 周常楷, 夏旻祺, 沈桢, 郭月, 吴强, 王喜章, 杨立军, 胡征. 钴取代多金属氧酸盐作为可溶性介质提升锂硫电池性能[J]. 化学学报, 2023, 81(6): 620-626. |
| [4] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [5] | 赵振新, 姚一琨, 陈佳骏, 牛蓉, 王晓敏. 一种高熵磷酸盐正极宿主实现高稳定性锂硫电池[J]. 化学学报, 2023, 81(5): 496-501. |
| [6] | 周俊粮, 赵振新, 武庭毅, 王晓敏. 多功能磷化铁碳布(FeP/CC)中间层高效催化多硫化物实现锂硫电池的高容量与高稳定性[J]. 化学学报, 2023, 81(4): 351-358. |
| [7] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [8] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [9] | 张冠华, 杨子涵, 马越. 混合工艺对氧化物/硫化物复合固态电解质电化学性能的影响[J]. 化学学报, 2023, 81(10): 1387-1393. |
| [10] | 张国强, 霍京浩, 王鑫, 郭守武. 基于P掺杂TiO2/C纳米管负极的高性能锂离子电容器[J]. 化学学报, 2023, 81(1): 6-13. |
| [11] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [12] | 梁世硕, 康树森, 杨东, 胡建华. 锂金属负极界面修饰及其在硫化物全固态电池中的应用[J]. 化学学报, 2022, 80(9): 1264-1268. |
| [13] | 马行宇, 孙晖, 李江, 刘之洋, 周红军. 基于Li-N2电池体系的“连续式”氮气还原合成氨[J]. 化学学报, 2022, 80(7): 861-866. |
| [14] | 何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征. 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022, 80(7): 896-902. |
| [15] | 毕文超, 张琳锋, 陈健, 田瑞雪, 黄昊, 姚曼. 单斜ZnP2负极材料的锂化机理及性能[J]. 化学学报, 2022, 80(6): 756-764. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||