化学学报 ›› 2022, Vol. 80 ›› Issue (6): 756-764.DOI: 10.6023/A21120552 上一篇 下一篇
研究论文
毕文超, 张琳锋, 陈健, 田瑞雪, 黄昊*( ), 姚曼*(
), 姚曼*( )
)
投稿日期:2021-12-09
发布日期:2022-07-07
通讯作者:
黄昊, 姚曼
基金资助:
Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang( ), Man Yao(
), Man Yao( )
)
Received:2021-12-09
Published:2022-07-07
Contact:
Hao Huang, Man Yao
Supported by:文章分享

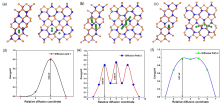
过渡金属磷化物电位低且比容量高, 是有发展前景的锂离子电池(LIBs)负极材料. 其中, ZnP2属于双活性负极材料, Zn与P都能与Li+发生反应, 储Li+性能更具有竞争力. 但是, 对于ZnP2的锂化机理及产物尚不明确. 采用第一性原理计算和电化学测试方法研究了ZnP2的电子性质和电化学性能, 通过理论计算和实验测试相结合阐述了ZnP2的锂化机制. 首先, 以密度泛函理论(DFT)计算揭示了ZnP2的锂化机理、Li+扩散路径、势垒和理论比容量(1477 mAh/g). 其次, 通过直流电弧等离子体法及固相烧结法合成ZnP2, 并测试其首圈放电曲线, 显示放电容量为1439 mAh/g, 与理论计算结果相近. 此外, 薄膜X射线衍射(XRD)检测最终产物成分为LiZn和Li3P, 与DFT计算结果一致.
毕文超, 张琳锋, 陈健, 田瑞雪, 黄昊, 姚曼. 单斜ZnP2负极材料的锂化机理及性能[J]. 化学学报, 2022, 80(6): 756-764.
Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang, Man Yao. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials[J]. Acta Chimica Sinica, 2022, 80(6): 756-764.
| Metal phosphide | P/M | Classification | Capacity/(mAh•g–1) |
|---|---|---|---|
| FeP2 | >1 (高磷比) | 单活性 | 1365[ |
| CuP2 | >1 (高磷比) | 单活性 | 1280[ |
| GeP5 | >1 (高磷比) | 双活性 | 2266[ |
| ZnP2 | >1 (高磷比) | 双活性 | 1477 |
| Ni2P | ≤1 (低磷比) | 单活性 | 542[ |
| Sn4P3 | ≤1 (低磷比) | 双活性 | 1255[ |
| Metal phosphide | P/M | Classification | Capacity/(mAh•g–1) |
|---|---|---|---|
| FeP2 | >1 (高磷比) | 单活性 | 1365[ |
| CuP2 | >1 (高磷比) | 单活性 | 1280[ |
| GeP5 | >1 (高磷比) | 双活性 | 2266[ |
| ZnP2 | >1 (高磷比) | 双活性 | 1477 |
| Ni2P | ≤1 (低磷比) | 单活性 | 542[ |
| Sn4P3 | ≤1 (低磷比) | 双活性 | 1255[ |
| Experimental/nm | Calculated/nm | Error/% |
|---|---|---|
| a=7.291×10–6 | a=7.31×10–6 | 0.38 |
| b=7.561×10–6 | b=7.604×10–6 | 0.57 |
| c=8.867×10–6 | c=8.886×10–6 | 0.21 |
| Experimental/nm | Calculated/nm | Error/% |
|---|---|---|
| a=7.291×10–6 | a=7.31×10–6 | 0.38 |
| b=7.561×10–6 | b=7.604×10–6 | 0.57 |
| c=8.867×10–6 | c=8.886×10–6 | 0.21 |
| Super cell | 1×1×1 | 2×1×1 | 2×2×1 | 2×2×2 | 3×3×3 |
|---|---|---|---|---|---|
| Eads/cell/eV | –101.254 | –101.252 | –101.253 | –101.254 | –101.240 |
| Super cell | 1×1×1 | 2×1×1 | 2×2×1 | 2×2×2 | 3×3×3 |
|---|---|---|---|---|---|
| Eads/cell/eV | –101.254 | –101.252 | –101.253 | –101.254 | –101.240 |
| Bond length/nm | ||||||
|---|---|---|---|---|---|---|
| Zn(1)—P(1) | Zn(1)—P(2) | Zn(2)—P(3) | Zn(2)—P(4) | P(1)—P(3) | P(2)—P(4) | |
| ZnP2 | 0.237 | 0.244 | 0.244 | 0.237 | 0.223 | 0.223 |
| LiZnP2 | 0.236 | 0.244 | 0.235 | 0.262 | 0.221 | 0.230 |
| Li3ZnP2 | 0.238 | 0.240 | 0.234 | 0.400 | 0.399 | 0.389 |
| Li5ZnP2 | 0.536 | 0.226 | 0.231 | 0.406 | 0.675 | 0.389 |
| Li7ZnP2 | 0.5639 | 0.461 | 0.250 | 0.411 | 0.529 | 0.403 |
| Bond length/nm | ||||||
|---|---|---|---|---|---|---|
| Zn(1)—P(1) | Zn(1)—P(2) | Zn(2)—P(3) | Zn(2)—P(4) | P(1)—P(3) | P(2)—P(4) | |
| ZnP2 | 0.237 | 0.244 | 0.244 | 0.237 | 0.223 | 0.223 |
| LiZnP2 | 0.236 | 0.244 | 0.235 | 0.262 | 0.221 | 0.230 |
| Li3ZnP2 | 0.238 | 0.240 | 0.234 | 0.400 | 0.399 | 0.389 |
| Li5ZnP2 | 0.536 | 0.226 | 0.231 | 0.406 | 0.675 | 0.389 |
| Li7ZnP2 | 0.5639 | 0.461 | 0.250 | 0.411 | 0.529 | 0.403 |







| [1] |
Choi, J. W.; Aurbach, D. Nat. Rev. Mater. 2016, 1, 16013.
doi: 10.1038/natrevmats.2016.13 |
| [2] |
Goodenough, J. B.; Park, K. S. J. Am. Chem. Soc. 2013, 135, 1167.
doi: 10.1021/ja3091438 pmid: 23294028 |
| [3] |
Lu, L.; Han, X.; Li, J.; Ouyang, M. J. Power Sources 2013, 226, 272.
doi: 10.1016/j.jpowsour.2012.10.060 |
| [4] |
Qiu, K.; Yan, M. X.; Zhao, S. W.; An, S. L.; Wang, W.; Jia, G. X. Acta Chim. Sinica 2021, 79, 1146. (in Chinese)
doi: 10.6023/A21040178 |
|
(邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄, 化学学报, 2021, 79, 1146.)
doi: 10.6023/A21040178 |
|
| [5] |
Tarascon, J. M.; Armand, M. Nature 2001, 414, 359.
doi: 10.1038/35104644 |
| [6] |
Zhou, X.; Liu, Q.; Jiang, C.; Ji, B.; Ji, X.; Tang, Y.; Cheng, H.-M. Angew. Chem., nt. Ed. 2019, 59, 3802.
|
| [7] |
Li, T. X.; Li, D. L.; Zhang, Q. B.; Gao, J. H.; Kong, X. Z.; Fan, X. Y.; Gou, L. Acta Chim. Sinica 2021, 79, 678. (in Chinese)
doi: 10.6023/A21010019 |
|
(李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾, 化学学报, 2021, 79, 678.)
doi: 10.6023/A21010019 |
|
| [8] |
Dahn, J. R.; Zheng, T.; Liu, Y.; Xue, J. Science 1995, 270, 590.
doi: 10.1126/science.270.5236.590 |
| [9] |
Zheng, S. Y.; Dong, F.; Pang, Y. P.; Han, P.; Yang, J. J. Inorg. Mater. 2020, 35, 1295. (in Chinese)
doi: 10.15541/jim20200134 |
|
(郑时有, 董飞, 庞越鹏, 韩盼, 杨俊和, 无机材料学报, 2020, 35, 1295.)
doi: 10.15541/jim20200134 |
|
| [10] |
Marino, C.; Debenedetti, A.; Fraisse, B.; Favier, F.; Monconduit, L. Electrochem. Commun. 2011, 13, 346.
doi: 10.1016/j.elecom.2011.01.021 |
| [11] |
Wang, S. L.; Yang, G. R.; SalmanNasir, M.; Wang, X. J.; Wang, J. N.; Yan, W. Acta Phys.-Chim. Sin. 2021, 37, 28. (in Chinese)
|
|
(王思岚, 杨国锐, SalmanNasir, Muhammad, 王筱珺, 王嘉楠, 延卫, 物理化学学报, 2021, 37, 28.)
|
|
| [12] |
Sun, J.; Zheng, G.; Lee, H.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Nano Lett. 2014, 14, 4573.
doi: 10.1021/nl501617j |
| [13] |
Kim, Y.; Park, Y.; Choi, A.; Choi, N. S.; Kim, J.; Lee, J.; Ji, H. R. Adv. Mater. 2013, 25, 3010.
doi: 10.1002/adma.201370143 |
| [14] |
Puziy, O.; Poddubnaya, A.; Martnez-Alonso, F.; Suarez-Garca; Tascon, J. M. D. Carbon 2002, 40, 1507.
doi: 10.1016/S0008-6223(01)00318-9 |
| [15] |
Jing, B.; Xi, B.; Mao, H.; Lin, Y.; Ma, X.; Feng, J.; Xiong, S. Adv. Mater. 2018, 1802310.
|
| [16] |
Wu, C.; Kopold, P.; Aken, P. A. V.; Maier, J.; Yu, Y. Adv. Mater. 2017, 29, 1604015.
doi: 10.1002/adma.201604015 |
| [17] |
Hou, B. H.; Wang, Y. Y.; Ning, Q. L.; Fan, C. Y.; Xi, X. T.; Yang, X. Nanoscale 2019, 11, 1304.
doi: 10.1039/C8NR08849G |
| [18] |
Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. ACS Nano 2017, 11, 11602.
doi: 10.1021/acsnano.7b06625 |
| [19] |
Pralong, V.; Souza, D.; Leung, K. T.; Nazar, L. F. Electrochem. Commun. 2002, 4, 516.
doi: 10.1016/S1388-2481(02)00363-6 |
| [20] |
Hall, J. W.; Membreno, N.; Jing, W.; Celio, H.; Jones, R. A. J. Am. Chem. Soc. 2012, 134, 5532.
doi: 10.1021/ja301173q |
| [21] |
Kim, K. H.; Hong, S. H. Adv. Energy Mater. 2021, 11, 2003609.
doi: 10.1002/aenm.202003609 |
| [22] |
Hayashi, A.; Inoue, A.; Tatsumisago, M. J. Power Sources 2009, 189, 669.
doi: 10.1016/j.jpowsour.2008.09.047 |
| [23] |
Kim, S. O.; Manthira, A. ACS Appl. Mater. Interfaces 2017, 9, 16221.
doi: 10.1021/acsami.7b02826 |
| [24] |
Chen, M.; Zhou, W.; Qi, M.; Yin, J.; Xia, X. J. Power Sources 2017, 342, 964.
doi: 10.1016/j.jpowsour.2017.01.014 |
| [25] |
Pfeiffer, H.; Tancret, F.; Brousse, T. Electrochim. Acta 2005, 50, 4763.
doi: 10.1016/j.electacta.2005.02.024 |
| [26] |
Lu, Y.; Wang, X.; Mai, Y.; Xiang, J.; Zhang, H.; Li, L.; Gu, C.; Tu, J.; Mao, S. X. J. Phys. Chem. C 2012, 116, 22217.
doi: 10.1021/jp3073987 |
| [27] |
Liu, J.; Sun, W.; Ran, Y.; Zhou, S.; Zhang, L.; Wu, A.; Huang, H.; Yao, M. Appl. Surf. Sci. 2021, 550, 149247.
doi: 10.1016/j.apsusc.2021.149247 |
| [28] |
Li, W.; Li, H.; Lu, Z.; Gan, L.; Ke, L.; Zhai, T.; Zhou, H. Energy Environ. Sci. 2015, 8, 3629.
doi: 10.1039/C5EE02524A |
| [29] |
Hwang, H.; Kim, M. G.; Kim, Y.; Martin, S. W.; Cho, J. Energy Environ. Sci. J. Mater. Chem. 2007, 3161.
|
| [30] |
Park, C.; Sohn, H. Chem. Mater. 2008, 20, 6319.
doi: 10.1021/cm800632f |
| [31] |
Liu, J.; Wu, A.; Tian, R.; Camacho, R. P.; Zhou, S.; Huang, S.; Yao, M. Mater. Today Energy 2020, 18, 100545.
|
| [32] |
Jain, A.; Ong, S. P.; Hautier, G.; Chen, W.; Richards, W. D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; Persson, K. A. APL Mater. 2013, 1, 011002.
doi: 10.1063/1.4812323 |
| [33] |
Fleet, M. E.; White, J. C. J. Mater. Res. 1986, 1, 187.
doi: 10.1557/JMR.1986.0187 |
| [34] |
Tian, R.; Liu, C.; Zhang, G.; Wu, A.; Yao, M.; Huang, H. Appl. Surf. Sci. 2021, 553, 149448.
doi: 10.1016/j.apsusc.2021.149448 |
| [35] |
Manju, M. S.; Thomas, S.; Lee, S. U.; Madam, A. K. Appl. Surf. Sci. 2020, 541, 148417.
doi: 10.1016/j.apsusc.2020.148417 |
| [36] |
Butler, K.; Gautam, G. S.; Canepa, P. NPJ Comput Mater. 2019, 5, 19.
doi: 10.1038/s41524-019-0160-9 |
| [37] |
Zhang, Z. F.; Yu, Q. Y.; Wu, L.; Sun, L. J.; Peng, J. H. J. Chongqing Univ. 2012, 35, 83. (in Chinese)
|
|
(张正富, 余秋雁, 伍林, 孙力军, 彭金辉, 重庆大学学报, 2012, 35, 83.)
|
|
| [38] |
Fleet, M. E.; Mowles, T. A. Acta Crystallogr. 1984, 40, 1778.
|
| [39] |
Aierken, Y.; Sevik, C.; Gulseren, O.; Peeters, F. M.; Cakir, D. J. Mater. Chem. A 2018, 6, 2337.
doi: 10.1039/C7TA09001C |
| [40] |
Li, P. J.; Zhou, W. W.; Tang, Y. H.; Zhang, H.; Shi, S. Q. Acta Phys. Sin. 2010, 6. (in Chinese)
|
|
(李沛娟, 周薇薇, 唐元昊, 张华, 施思齐, 物理学报, 2010, 6.)
|
|
| [41] |
Fan, C. L.; Cheng, X. L.; Zhang, H. Phys. Status Solidi 2010, 246, 77.
doi: 10.1002/pssb.200844007 |
| [42] |
Henkelman, G.; Uberuaga, B. P. J. Phys. Chem. C 2000, 113, 9901.
|
| [43] |
Harper, A. F.; Evans, M. L.; Darby, J. P.; Bora, K.; Koer, C. P.; Nelson, J. R.; Morris, A. J. Johnson Matthey Technol. Rev. 2020, 64, 103.
doi: 10.1595/205651320X15742491027978 |
| [44] |
Lin, C. J.; Zheng, F.; Zhu, Z. Z. Acta Phys. Sin. 2019, 68, 8. (in Chinese)
|
|
(林传金, 郑锋, 朱梓忠, 物理学报, 2019, 68, 8.)
|
|
| [45] |
Chen, H.; Hua, Y.; Luo, N.; He, X.; Li, Y.; Zhang, Y.; Chen, W.; Huang, S. J. Phys. Chem. C 2020, 124, 7031.
doi: 10.1021/acs.jpcc.0c00103 |
| [46] |
Zhao, S.; Kang, W.; Xue, J. J. Mater. Chem. A 2014, 2, 19046.
doi: 10.1039/C4TA04368E |
| [47] |
Hardikar, R. P.; Das, D.; Han, S. S.; Lee, K. R.; Singh, A. K. Phys. Chem. Chem. Phys. 2014, 16, 16502.
doi: 10.1039/c4cp01412j pmid: 24986702 |
| [48] |
Zhang, W.; Liu, S.; Chen, J.; Hu, F.; Wang, X.; Huang, H.; Yao, M. ACS Appl. Mater. Interfaces 2021, 13, 22341.
doi: 10.1021/acsami.1c02470 |
| [49] |
Fang, Y.; Zhang, Y.; Miao, C.; Zhu, K.; Chen, Y.; Du, F.; Yin, J.; Ye, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Nano-micro. Lett. 2020, 12, 128.
|
| [50] |
Du, F.; Jin, X.; Chen, J.; Hua, Y.; Cao, M.; Zhang, L.; Li, J.; Zhang, L.; Jin, J.; Wu, C. J. Nanopart. Res. 2014, 16, 1.
|
| [51] |
Kim, Y. U.; Lee, C. K.; Kang, T. J. Electrochem. Soc. 2004, 151, A933.
doi: 10.1149/1.1738679 |
| [52] |
Berland, K.; Hyldgaard, P. Phys. Rev. B 2014, 89, 035412.
doi: 10.1103/PhysRevB.89.035412 |
| [53] |
Kresse, G. G.; Furthmüller, J. J. Phys. Rev. B 1996, 54, 11169.
doi: 10.1103/physrevb.54.11169 pmid: 9984901 |
| [54] |
Chl, P. Phys. Rev. B 1994, 50, 1795.
|
| [55] |
Paier, J.; Hirschl, R.; Marsman, M.; Georg, K. J. Phys. Chem. C 2005, 122, 234102.
|
| [56] |
Broderick, S. R.; Rajan, K. Europhys. Lett. 2011, 95, 57005.
doi: 10.1209/0295-5075/95/57005 |
| [57] |
Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272.
doi: 10.1107/S0021889811038970 |
| [1] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [2] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [3] | 杨洁, 凌琳, 李玉学, 吕龙. 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023, 81(4): 328-337. |
| [4] | 王晓钰, 张渝, 马磊, 魏良明. 锂离子电池硅基负极粘结剂发展现状[J]. 化学学报, 2019, 77(1): 24-40. |
| [5] | 王玲, 杨国锐, 王嘉楠, 王思岚, 彭生杰, 延卫. 静电纺丝在钠离子电池中的应用研究进展[J]. 化学学报, 2018, 76(9): 666-680. |
| [6] | 李志伟, 仲佳亮, 陈楠楠, 薛兵, 米红宇. 氮掺杂多孔炭片的模板法制备及其储锂性能研究[J]. 化学学报, 2018, 76(3): 209-214. |
| [7] | 母伟花, 马瑶, 方德彩, 王蓉, 张海娜. 1-碘-2-锂-邻碳硼烷与环戊二烯衍生物的类Diels-Alder反应的理论研究[J]. 化学学报, 2018, 76(1): 55-61. |
| [8] | 向兴德, 卢艳莹, 陈军. 钠离子电池先进功能材料的研究进展[J]. 化学学报, 2017, 75(2): 154-162. |
| [9] | 杜进, 林宁, 钱逸泰. Si/石墨复合负极材料的制备方法研究进展[J]. 化学学报, 2017, 75(2): 147-153. |
| [10] | 王蕾, 赵冬冬, 刘旭, 于鹏, 付宏刚. 水热法合成氧化亚钴纳米粒子/石墨烯复合材料及其储锂性能研究[J]. 化学学报, 2017, 75(2): 231-236. |
| [11] | 杨一诺, 张琪, 石景, 傅尧. Mn(I)催化亚胺和炔烃脱氢偶联反应的机理研究[J]. 化学学报, 2016, 74(5): 422-428. |
| [12] | 童震坤, 方姗, 郑浩, 张校刚. 锗酸锌纳米棒@石墨烯复合负极材料的制备及储锂性质[J]. 化学学报, 2016, 74(2): 185-190. |
| [13] | 罗飞, 郑杰允, 褚赓, 刘柏男, 张素林, 李泓, 陈立泉. 高容量金属镓薄膜和粉体作为锂离子电池负极材料的自修复行为研究[J]. 化学学报, 2015, 73(8): 808-814. |
| [14] | 叶亚, 朱婧怡, 姚依男, 王雨果, 吴平, 唐亚文, 周益明, 陆天虹. 在多元醇体系中一锅法合成具有良好储锂性能的介孔碳-锡复合材料[J]. 化学学报, 2015, 73(2): 151-155. |
| [15] | 吕之阳, 冯瑞, 赵进, 范豪, 徐丹, 吴强, 杨立军, 陈强, 王喜章, 胡征. 高倍率锂离子电池负极材料:氮掺杂碳纳米笼[J]. 化学学报, 2015, 73(10): 1013-1017. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||