化学学报 ›› 2021, Vol. 79 ›› Issue (12): 1477-1480.DOI: 10.6023/A21090432 上一篇 下一篇
研究通讯
投稿日期:2021-09-16
发布日期:2021-11-01
通讯作者:
乔保坤, 江智勇
基金资助:
Hongshao Jiaa, Baokun Qiaoa( ), Zhiyong Jiangb(
), Zhiyong Jiangb( )
)
Received:2021-09-16
Published:2021-11-01
Contact:
Baokun Qiao, Zhiyong Jiang
Supported by:文章分享
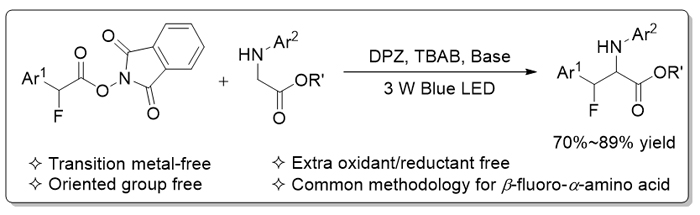
通过可见光驱动光氧化还原催化, 发展了一种新颖、便利的β-氟代-α-氨基酸衍生物的合成方法. 以非金属的二氰基吡嗪衍生物(DPZ)为光催化剂, 以易于制备的N-芳基甘氨酸酯和芳基乙酸氧化还原酯为原料, 通过单电子氧化还原分别生成酯基取代α-氨烷基自由基及α-氟代苄基自由基. 经过高反应活性自由基的交叉偶联, 高产率地得到目标产物. 该方法由于氧化还原中性反应途径而无需额外的氧化剂或还原剂, 且属于绿色、可持续的有机催化合成策略.
贾红绍, 乔保坤, 江智勇. 光氧化还原催化自由基偶联合成β-氟代-α-氨基酸衍生物[J]. 化学学报, 2021, 79(12): 1477-1480.
Hongshao Jia, Baokun Qiao, Zhiyong Jiang. Photoredox Catalytic Radical Coupling to Access β-Fluoro α-Amino Acid Derivatives[J]. Acta Chimica Sinica, 2021, 79(12): 1477-1480.
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 89 |
| 2 | 4DPAIPN instead of DPZ | 76 |
| 3 | Ir(III)c complex instead of DPZ | 83 |
| 4 | CH3CN instead of CPME | 81 |
| 5 | toluene instead of CPME | 66 |
| 6 | THF instead of CPME | 73 |
| 7 | 1b instead of 1a | 69 |
| 8 | 1c instead of 1a | 77 |
| 9 | no TBAB | <5 |
| 10 | no NaH2PO4 | <5 |
| 11 | no DPZ | 0d |
| 12 | no light | 0d |
| 13 | Under air | 0d |
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 89 |
| 2 | 4DPAIPN instead of DPZ | 76 |
| 3 | Ir(III)c complex instead of DPZ | 83 |
| 4 | CH3CN instead of CPME | 81 |
| 5 | toluene instead of CPME | 66 |
| 6 | THF instead of CPME | 73 |
| 7 | 1b instead of 1a | 69 |
| 8 | 1c instead of 1a | 77 |
| 9 | no TBAB | <5 |
| 10 | no NaH2PO4 | <5 |
| 11 | no DPZ | 0d |
| 12 | no light | 0d |
| 13 | Under air | 0d |
| [1] |
For selected reviews, see: (a) Yoder, N. C.; Kumar, K. Chem. Soc. Rev. 2002, 31, 335.
doi: 10.1039/b201097f pmid: 22130572 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C pmid: 22130572 |
|
|
(c) Salwiczek, M. Chem. Soc. Rev. 2012, 41, 2135.
doi: 10.1039/c1cs15241f pmid: 22130572 |
|
|
(d) Tan, F.; Xiao, W. Acta Chim. Sinica 2015, 73, 85 (in Chinese).
doi: 10.6023/A14120860 pmid: 22130572 |
|
|
( 谭芬, 肖文精, 化学学报, 2015, 73, 85.)
doi: 10.6023/A14120860 pmid: 22130572 |
|
| [2] |
For selected reviews, see: (a) Eriksson, T.; Bjöurkman, S.; Roth, B.; Fyge, Å.; Höuglund, P. Chirality 1995, 7, 44.
pmid: 25843369 |
|
(b) Okarvi, S. Eur. J. Nucl. Med. 2001, 28, 929.
pmid: 25843369 |
|
|
(c) Yu, W.; McConathy, J.; Williams, L.; Camp, V. M.; Malveaux, E. J.; Zhang, Z.; Olson, J. J.; Goodman, M. M. J. Med. Chem. 2009, 53, 876.
doi: 10.1021/jm900556s pmid: 25843369 |
|
|
(d) Buer, B. C.; Marsh, E. N. G. Protein Sci. 2012, 21, 453.
doi: 10.1002/pro.2030 pmid: 25843369 |
|
|
(e) Huang, C.; McConathy, J. Curr. Top. Med. Chem. 2013, 13, 871.
doi: 10.2174/1568026611313080002 pmid: 25843369 |
|
|
(f) Yu, W.; McConathy, J.; Olson, J. J.; Goodman, M. M. Nucl. Med. Biol. 2015, 42, 8.
doi: 10.1016/j.nucmedbio.2014.07.002 pmid: 25843369 |
|
|
(g) Bouhlel, A.; Zhou, D.; Li, A.; Yuan, L.; Rich, K. M.; McConathy, J. J. Med. Chem. 2015, 58, 3817.
doi: 10.1021/jm502023y pmid: 25843369 |
|
| [3] |
(a) Wade, T. N. J. Org. Chem. 1980, 45, 5328.
doi: 10.1021/jo01314a023 |
|
(b) Hart, B. P.; Coward, J. K. Tetrahedron Lett. 1993, 34, 4917.
doi: 10.1016/S0040-4039(00)74045-7 |
|
|
(c) Kukhar, V. P. J. Fluorine Chem. 1994, 69, 199.
doi: 10.1016/0022-1139(94)03131-2 |
|
|
(d) Shi, G.-Q.; Zhao, Z.-Y.; Zhang, X.-B. J. Org. Chem. 1995, 60, 6608.
doi: 10.1021/jo00125a060 |
|
|
(e) Ayi, A. I.; Guedj, R.; Septe, B. J. Fluorine Chem. 1995, 73, 165.
doi: 10.1016/0022-1139(94)03224-N |
|
| [4] |
(a) Zhu, R.-Y.; Tanaka, K.; Li, G.-C.; He, J.; Fu, H.-Y.; Li, S.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 7067.
doi: 10.1021/jacs.5b04088 pmid: 26067591 |
|
(b) Zhang, Q.; Yin, X.-S.; Chen, K.; Zhang, S.-Q.; Shi, B.-F. J. Am. Chem. Soc. 2015, 137, 8219.
doi: 10.1021/jacs.5b03989 pmid: 26067591 |
|
|
(c) Bume, D. D.; Pitts, C. R.; Jokhai, R. T.; Lectka, T. Tetrahedron 2016, 72, 6031.
doi: 10.1016/j.tet.2016.08.018 pmid: 26067591 |
|
| [5] |
For selected reviews, see: (a) Xuan, J.; Xiao, W.-J. Angew. Chem. Int. Ed. 2012, 51, 6828.
doi: 10.1002/anie.201200223 |
|
(b) Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044.
doi: 10.1039/C5CS00655D |
|
|
(c) Stephenson, C. R. J.; Yoon, T. P.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry, John Wiley & Sons, New Jersey, 2018.
|
|
|
(d) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433.
doi: 10.1021/acs.chemrev.6b00657 |
|
|
(e) Liu, Q.; Wu, L.-Z. Natl. Sci. Rev. 2017, 4, 359.
doi: 10.1093/nsr/nwx039 |
|
|
(f) Qiao, B.; Jiang, Z. ChemPhotoChem 2018, 2, 703.
doi: 10.1002/cptc.v2.8 |
|
|
(g) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 |
|
|
(h) Ren, L.; Ran, M.; He, J.; Qian, Y.; Yao, Q. Chin. J. Org. Chem. 2019, 39, 1583 (in Chinese).
doi: 10.6023/cjoc201812042 |
|
|
( 任林静, 冉茂刚, 何佳芯, 钱燕, 姚秋丽, 有机化学, 2019, 39, 1583.)
doi: 10.6023/cjoc201812042 |
|
| [6] |
For selected reviews, see: (a) Yin, Y.; Zhao, X.; Qiao, B.; Jiang, Z. Org. Chem. Front. 2020, 7, 1283.
doi: 10.1039/D0QO00276C |
|
(b) Lv, X.; Xu, H.; Yin, Y.; Zhao, X.; Jiang, Z. Chin. J. Chem. 2020, 38, 1480.
doi: 10.1002/cjoc.v38.12 |
|
|
(c) Srivastava, V.; Singh, P. K.; Srivastava, A.; Sinha, S.; Singh, P. P. Photochem 2021, 1, 237.
doi: 10.3390/photochem1020014 |
|
| [7] |
For selected reviews, see: (a) Li, J.; Kong, M.; Qiao, B.; Lee, R.; Zhao, X.; Jiang, Z. Nat. Commun. 2018, 9, 2445.
doi: 10.1038/s41467-018-04885-3 |
|
(b) Liu, Y.; Liu, X.; Li, J.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2018, 9, 8094.
doi: 10.1039/C8SC02948B |
|
|
(c) Yin, Y.; Dai, Y.; Jia, H.; Li, J.; Bu, L.; Qiao, B.; Zhao, X.; Jiang, Z. J. Am. Chem. Soc. 2018, 140, 6083.
doi: 10.1021/jacs.8b01575 |
|
| [8] |
(a) Wang, C.; Guo, M.; Qi, R.; Shang, Q.; Liu, Q.; Wang, S.; Zhao, L.; Wang, R.; Xu, Z. Angew. Chem. Int. Ed. 2018, 57, 15841.
doi: 10.1002/anie.201809400 |
|
(b) Zhu, Z.; Xiao, L.; Xie, Z.; Le, Z. Chin. J. Org. Chem. 2019, 39, 2345 (in Chinese).
doi: 10.6023/cjoc201903006 |
|
|
( 祝志强, 肖利金, 谢宗波, 乐长高, 有机化学, 2019, 39, 2345.)
doi: 10.6023/cjoc201903006 |
|
| [9] |
For selected reviews, see: (a) Cheng, W.-M.; Shang, R.; Fu, Y. ACS Catal. 2016, 7, 907.
doi: 10.1021/acscatal.6b03215 pmid: 28841018 |
|
(b) Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K. Science 2017, 357, 283.
doi: 10.1126/science.aan3679 pmid: 28841018 |
|
|
(c) Zhao, W.; Wurz, R. P.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2017, 139, 12153.
doi: 10.1021/jacs.7b07546 pmid: 28841018 |
|
|
(d) Mao, R.; Frey, A.; Balon, J.; Hu, X. Nat. Catal. 2018, 1, 120.
doi: 10.1038/s41929-017-0023-z pmid: 28841018 |
|
| [10] |
Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Org. Lett. 2018, 20, 6298.
doi: 10.1021/acs.orglett.8b02791 |
| [11] |
(a) Liu, X.; Ye, X.; Bureš, F.; Liu, H.; Jiang, Z. Angew. Chem. Int. Ed. 2015, 54, 11443.
doi: 10.1002/anie.v54.39 |
|
(b) Lin, L.; Bai, X.; Ye, X.; Zhao, X.; Tan, C.-H.; Jiang, Z. Angew. Chem. Int. Ed. 2017, 56, 13842.
doi: 10.1002/anie.201707899 |
|
|
(c) Shao, T.; Jiang, Z. Acta Chim. Sinica 2017, 75, 80 (in Chinese).
doi: 10.6023/A16090496 |
|
|
( 邵天举, 江智勇, 化学学报, 2017, 75, 80.)
|
|
|
(d) Hou, M.; Lin, L.; Chai, X.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2019, 10, 6629.
doi: 10.1039/C9SC02000D |
|
|
(e) Qiao, B.; Li, C.; Zhao, X.; Yin, Y.; Jiang, Z. Chem. Commun. 2019, 55, 7534.
doi: 10.1039/C9CC03661J |
|
|
(f) Shao, T.; Li, Y.; Ma, N.; Li, C.; Chai, G.; Zhao, X.; Qiao, B.; Jiang, Z. iScience 2019, 16, 410.
doi: 10.1016/j.isci.2019.06.007 |
|
|
(g) Yin, Y.; Li, Y.; Gonçalves, T. P.; Zhan, Q.; Wang, G.; Zhao, X.; Qiao, B.; Huang, K.-W.; Jiang, Z. J. Am. Chem. Soc. 2020, 142, 19451.
doi: 10.1021/jacs.0c08329 |
|
|
(h) Kong, M.; Tan, Y.; Zhao, X.; Qiao, B.; Tan, C.-H.; Cao, S.; Jiang, Z. J. Am. Chem. Soc. 2021, 143, 4024.
doi: 10.1021/jacs.1c01073 |
|
| [12] |
(a) Oscarson, S. Protective Group Strategies, CRC Press, Taylor Francis Group, Boca Raton, 2006.
|
|
(b) Wuts, P. G.; Greene, T. W. Greene's Protective Groups in Organic Synthesis, John Wiley & Sons, New Jersey, 2006.
|
|
| [13] |
(a) Walling, C. Free Radicals in Solution, John Wiley & Sons, Inc., New York, 1957.
pmid: 23121090 |
|
(b) Curran, D. P.; Porter, N. A.; Giese, B. Stereochemistry of Radical Reactions:Concepts, Guidelines and Synthetic Applications, Wiley-VCH Verlag, Weinheim, Germany, 1996.
pmid: 23121090 |
|
|
(c) Roberts, B. P. Chem. Soc. Rev. 1999, 28, 25.
doi: 10.1039/a804291h pmid: 23121090 |
|
|
(d) Hata, S.; Sibi, M. P. Addition of Free Radicals to Carbon-Carbon Multiple Bonds, In Stereoselective Synthesis, Vol. 1, Eds.: De Vries, J. G.; Molander, G. A.; Evans, P. A., Georg Thieme Verlag, Stuttgart, Germany, 2011.
pmid: 23121090 |
|
|
(e) Wille, U. Chem. Rev. 2013, 113, 813.
doi: 10.1021/cr100359d pmid: 23121090 |
|
| [14] |
See the Supporting Information for details.
|
| [1] | 张洪浩, 俞寿云. 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019, 77(9): 832-840. |
| [2] | 吴自俊, 汪舰. 可见光氧化还原催化下α-酮酸对对位亚甲基醌(p-QMs)脱羧/1,6-共轭加成反应[J]. 化学学报, 2017, 75(1): 74-79. |
| [3] | 荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波. 可见光促进下氟烷基砜对芳基烯烃的自由基氟烷基化反应[J]. 化学学报, 2017, 75(1): 105-109. |
| [4] | 周泉泉, 刘丹, 肖文精, 陆良秋. 可见光光氧化还原催化的三级胺α位C-H氰基化反应[J]. 化学学报, 2017, 75(1): 110-114. |
| [5] | 谭芬, 肖文精. 可见光促进的氧化还原催化反应在天然产物全合成中的应用[J]. 化学学报, 2015, 73(2): 85-89. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||