化学学报 ›› 2019, Vol. 77 ›› Issue (9): 832-840.DOI: 10.6023/A19050177 上一篇 下一篇
所属专题: 有机自由基化学
综述
投稿日期:2019-05-14
发布日期:2019-07-16
通讯作者:
俞寿云
E-mail:yushouyun@nju.edu.cn
作者简介:张洪浩, 1991年出生于江苏扬州, 2009年和2013年于江苏师范大学先后获得学士和硕士学位, 2013年至今在俞寿云教授指导下攻读博士学位. 其研究兴趣主要是可见光促进的不对称催化反应.|俞寿云, 1978年11月出生于江苏南京. 2001年获南京大学理学学士学位. 2006年获中国科学院上海有机化学研究所理学博士学位(导师为马大为研究员). 2006年到2007年留所任助理研究员. 2007年-2010年在美国宾夕法尼亚大学进行博士后研究(合作导师Jeffrey W. Bode教授). 2010年9月被聘为南京大学化学化工学院副教授, 博士生导师. 2016年起任南京大学化学化工学院教授. 曾获2012年Thieme Chemistry Journals Award, 2007年国家自然科学二等奖(排名第四)和2005年上海市科技进步一等奖(排名第四)等奖项.
基金资助:
Zhang, Hong-Hao, Yu, Shouyun*( )
)
Received:2019-05-14
Published:2019-07-16
Contact:
Yu, Shouyun
E-mail:yushouyun@nju.edu.cn
Supported by:文章分享
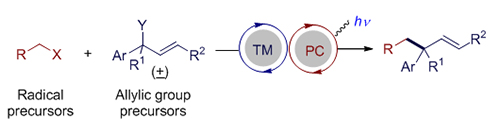
过渡金属催化的烯丙基取代反应是一类重要且实用的有机化学反应, 可以立体选择性地高效构建碳-碳键和碳-杂键. 可见光氧化还原催化可以利用绿色清洁的可见光能源在较为温和的条件下产生自由基或者自由基离子等高反应活性的反应中间体, 被广泛地应用于有机合成中, 逐渐发展成为一种重要的合成工具. 鉴于烯丙基取代反应的重要性, 过渡金属与光氧化还原协同催化的烯丙基取代反应逐渐引起化学家的兴趣. 该协同催化的策略可以实现单一过渡金属催化难以实现的烯丙基取代反应, 反应的区域选择性和立体选择性也体现出不同的特点, 有望发展成为单一金属催化的烯丙基取代反应的重要补充. 本文综述了近年来不同过渡金属与可见光氧化还原协同催化的烯丙基取代反应的研究进展.
张洪浩, 俞寿云. 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019, 77(9): 832-840.
Zhang, Hong-Hao, Yu, Shouyun. Advances on Transition Metals and Photoredox Cooperatively Catalyzed Allylic Substitutions[J]. Acta Chimica Sinica, 2019, 77(9): 832-840.
| [1] |
(a) Tsuji, J.; Takahashi, H.; Morikawa, M . Tetrahedron Lett. 1965, 6, 4387.
doi: 10.1016/S0040-4039(00)71674-1 |
|
(b) Tsuji, J . Acc. Chem. Res. 1969, 2, 144.
doi: 10.1016/S0040-4039(00)71674-1 |
|
| [2] |
Trost, B. M.; Strege, P. E. J. Am. Chem. Soc. 1977, 99, 1649.
doi: 10.1021/ja00447a064 |
| [3] |
(a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395.
doi: 10.1021/cr9409804 |
|
(b) Trost B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
doi: 10.1021/cr9409804 |
|
|
(c) Trost, B. M.; Machacek, M. R.; Aponick, A. Acc. Chem. Res. 2006, 39, 747.
doi: 10.1021/cr9409804 |
|
|
(d) Trost, B. M. Org. Process Res. Dev. 2012, 16, 185. 185.
doi: 10.1021/cr9409804 |
|
| [4] |
(a) Wang, Y-N.; Lu, L.-Q.; Xiao, W.-J. Chem. Asian J. 2018, 13, 2174.
doi: 10.6023/A16020078 |
|
(b) Tang, H.; Huo, X.; Meng, Q.; Zhang, W. Acta Chim. Sinica 2016, 74, 219 (in Chinese).
doi: 10.6023/A16020078 |
|
|
(汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.)
doi: 10.6023/A16020078 |
|
| [5] |
(a) Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461.
doi: 10.1021/ar100047x |
|
(b) Cheng, Q.; Tu, H. F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. . Chem Rev. 2019, 119, 1855.
doi: 10.1021/ar100047x |
|
|
(c) Deng, Y.; Yang, W.; Yang, X.; Yang, D.Chin. J. Org. Chem. 2017, 37, 3039 (in Chinese).
doi: 10.1021/ar100047x |
|
|
(邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.)
doi: 10.1021/ar100047x |
|
| [6] |
(a) Alexakis, A.; Bäckvall, J. E.; Krause, N.; Pàmies, O.; Diéguez, M. . Chem Rev. 2008, 108, 2796.
doi: 10.1021/cr0683515 |
|
(b) Yorimitsu, H.; Oshima, K. Angew. Chem., Int. Ed. 2005, 44, 4435.
doi: 10.1021/cr0683515 |
|
| [7] |
Zhang, H.; Gu, Q.; You, S.-L . Chin. J. Org. Chem. 2019, 39, 15 (in Chinese).
doi: 10.6023/cjoc201809037 |
|
( 张慧君, 顾庆, 游书力 , 有机化学, 2019, 39, 15.)
doi: 10.6023/cjoc201809037 |
|
| [8] |
(a) Turnbull, B. W. H.; Evans, P. A. J. Org. Chem. 2018, 83, 11463.
doi: 10.1021/acs.joc.8b00583 |
|
(b) Thoke, M. B.; Kang, Q. Synthesis 2019, DOI: 10. 1055/s-0037-1611784.
doi: 10.1021/acs.joc.8b00583 |
|
| [9] |
Bruneau, C.; Renaud, J.-L.; Demerseman, B. Chem. Eur. J. 2006, 12, 5178.
doi: 10.1002/(ISSN)1521-3765 |
| [10] |
(a) Trost, B. M.; Verhoeven, T. R. J. Org. Chem. 1976, 41, 3215.
doi: 10.1021/jo00881a039 |
|
(b) Matsushita, H.; Negishi, E. J. Chem. Soc. Chem. Commun. 1982, 160.
doi: 10.1021/jo00881a039 |
|
|
(c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
doi: 10.1021/jo00881a039 |
|
|
(d) Weaver, J. D.; Recio, III, A.; Grenning, A. J.; Tunge, J. A. Chem. Rev. 2011, 111, 1846.
doi: 10.1021/jo00881a039 |
|
|
(e) Yu, Y.-N.; X, M.-H. Acta Chim. Sinica 2017, 75, 655 (in Chinese). (于月娜, 徐明华, 化学学报, 2017, 75, 655.)
doi: 10.1021/jo00881a039 |
|
|
(f) Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synjournal, Ed.: Kazmaier, U., Springer, Heidelberg, 2012.
doi: 10.1021/jo00881a039 |
|
| [11] |
For reviews on this topic, see: (a) Harutyunyan, S. R.; den Hartog, T.; Geurts, K.; Minnaard, A. J.; Feringa, B. L. Chem. Rev. 2008, 108, 2824.
doi: 10.1021/cr068424k |
|
(b) Alexakis, A.; Backvall, J. E.; Krause, N.; Pàmies, O.; Diéguez, M . Chem. Rev. 2008, 108, 2796.
doi: 10.1021/cr068424k |
|
|
(c) Teichert, J. F.; Ferin-ga, B. L. Angew. Chem., Int. Ed. 2010, 49, 2486.
doi: 10.1021/cr068424k |
|
|
(d) Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587.
doi: 10.1021/cr068424k |
|
|
(e) Hartwig, J. F.; Pouy, M. J. Top. Organomet. Chem. 2011, 34, 169.
doi: 10.1021/cr068424k |
|
|
(f) Schäfer, P.; Sidera, M.; Palacin, T.; Fletcher, S. P. Chem. Commun. 2017, 53, 12499.
doi: 10.1021/cr068424k |
|
| [12] |
(a) Zheng, W.-H.; Zheng, B.-H.; Zhang, Y.; Hou, X.-L. J. Am. Chem. Soc. 2007, 129, 7718.
doi: 10.1021/ja071098l |
|
(b) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2008, 130, 14092.
doi: 10.1021/ja071098l |
|
|
(c) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2009, 131, 12056.
doi: 10.1021/ja071098l |
|
|
(d) Zhang, P.; Brozek, L. A.; Morken, J. P. J. Am. Chem. Soc. 2010, 132, 10686.
doi: 10.1021/ja071098l |
|
|
(e) Chen, J.-P.; Ding, C.-H.; Liu, W.; Hou, X.-L.; Dai, L.-X. J. Am. Chem. Soc. 2010, 132, 15493.
doi: 10.1021/ja071098l |
|
|
(f) Zhang, P.; Le, H.; Kyne, R. E.; Morken, J. P. J. Am. Chem. Soc. 2011, 133, 9716.
doi: 10.1021/ja071098l |
|
|
(g) Trost, B. M.; Thaisrivongs, D. A.; Hartwig, J. J. Am. Chem. Soc. 2011, 133, 12439.
doi: 10.1021/ja071098l |
|
|
(h) Chen, J.-P.; Peng, Q.; Lei, B.-L.; Hou, X.-L.; Wu, Y.-D. J. Am. Chem. Soc. 2011, 133, 14180.
doi: 10.1021/ja071098l |
|
|
(i) Brozek, L. A.; Ardolino, M. J.; Morken, J. P. J. Am. Chem. Soc. 2011, 133, 16778.
doi: 10.1021/ja071098l |
|
|
(j) Ardolino, M. J.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 7092.
doi: 10.1021/ja071098l |
|
|
(k) Niyomchon, S.; Audisio, D.; Luparia, M.; Maulide, N. Org. Lett. 2013, 15, 2318.
doi: 10.1021/ja071098l |
|
|
(l) Misale, A.; Niyomchon, S.; Luparia, M.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 7068.
doi: 10.1021/ja071098l |
|
|
(m) Mao, J.; Zhang, J.; Jiang, H.; Bellomo, A.; Zhang, M.; Gao, Z.; Dreher, S. D.; Walsh, P. J. Angew. Chem., Int. Ed. 2016, 55, 2526.
doi: 10.1021/ja071098l |
|
|
(n) Murakami, R.; Sano, K.; Iwai, T.; Taniguchi, T.; Monde, K.; Sawamura, M. Angew. Chem., Int. Ed. 2018, 57, 9465.
doi: 10.1021/ja071098l |
|
| [13] |
(a) Butt, N.; Yang, G.; Zhang, W. Chem . Rec. 2016, 16, 2687.
doi: 10.6023/A18060237 |
|
(b) Afewerki, S.; Córdova, A. Chem . Rev. 2016, 116, 13512.
doi: 10.6023/A18060237 |
|
|
(c) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838 (in Chinese).
doi: 10.6023/A18060237 |
|
|
(张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
|
(d) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
doi: 10.6023/A18060237 |
|
|
(e) Fu, J.; Huo, X.; Li, B.; Zhang, W. Org. Biomol. Chem. 2017, 15, 9747.
doi: 10.6023/A18060237 |
|
| [14] |
For reviews on photoredox catalysis, see: (a) Narayanam, J. M. R.; Ste-phenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102.
doi: 10.1039/B913880N |
|
(b) Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1039/B913880N |
|
|
(c) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1039/B913880N |
|
|
(d) Dai, X.-J.; Xu, X.-L.; Li, X.-N. Chin. J. Org. Chem. 2013, 33, 2046 (in Chinese).
doi: 10.1039/B913880N |
|
|
(戴小军, 许孝良, 李小年, 有机化学, 2013, 33, 2046.)
doi: 10.1039/B913880N |
|
|
(e) Schultz, D. M.; Yoon, T. P. Science 2014, 343, 1239176.
doi: 10.1039/B913880N |
|
|
(f) Brim-ioulle, R.; Lenhart, D.; Maturi, M. M.; Bach, T. Angew. Chem., Int. Ed. 2015, 54, 3872.
doi: 10.1039/B913880N |
|
|
(g) Tan, F.; Xiao, W. Acta Chim. Sinica 2015, 73, 85 (in Chinese).
doi: 10.1039/B913880N |
|
|
(谭芬, 肖文精, 化学学报, 2015, 73, 85.)
doi: 10.1039/B913880N |
|
|
(h) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1039/B913880N |
|
|
(i) Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850.
doi: 10.1039/B913880N |
|
| [15] |
For selected examples on palladium metallaphotoredox catalysis, see: (a) Kalyani, D.; McMurtrey, K. B.; Neufeldt, S. R.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18566.
doi: 10.1021/ja208068w |
|
(b) Neufeldt, S. R.; Sanford, M. S. Adv. Synth. Catal. 2012, 354, 3517.
doi: 10.1021/ja208068w |
|
|
(c) Zoller, J.; Fabry, D. C.; Ronge, M. A.; Rueping, M. . Angew. Chem., Int. Ed. 2014, 53, 13264.
doi: 10.1021/ja208068w |
|
|
(d) Mori, K.; Kawashima, M.; Yamashita, H . Chem. Commun. 2014, 50, 14501.
doi: 10.1021/ja208068w |
|
|
(e) Choi, S.; Chatterjee, T.; Choi, W. J.; You, Y.; Cho, E. J . ACS Catal. 2015, 5, 4796.
doi: 10.1021/ja208068w |
|
|
(f) Zhou, C.; Li, P.; Zhu, X.; Wang, L . Org. Lett. 2015, 17, 6198.
doi: 10.1021/ja208068w |
|
|
(g) Cheng, W.-M.; Shang, R.; Yu, H.-Z.; Fu, Y . Chem. Eur. J. 2015, 21, 13191.
doi: 10.1021/ja208068w |
|
|
(h) Liu, K.; Zou, M.; Lei, A . J. Org. Chem. 2016, 81, 7088.
doi: 10.1021/ja208068w |
|
|
(i) Kärkäs, M. D.; Bosque, I.; Matsuura, B. S.; Stephenson, C. R. J . Org. Lett. 2016, 18, 5166.
doi: 10.1021/ja208068w |
|
|
(j) Shimomaki, K.; Murata, K.; Martin, R.; Iwasawa, N . J. Am. Chem. Soc. 2017, 139, 9467.
doi: 10.1021/ja208068w |
|
|
(k) Kato, S.; Saga, Y.; Kojima, M.; Fuse, H.; Matsunaga, S.; Fukatsu, A.; Kondo, M.; Masaoka, S.; Kanai, M . J. Am. Chem. Soc. 2017, 139, 2204.
doi: 10.1021/ja208068w |
|
| [16] |
For selected examples on nickel metallaphotoredox catalysis, see: (a) Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; MacMillan, D. W. C. Science 2014, 345, 437.
doi: 10.1002/anie.201504963 |
|
(b) Tellis, J. C.; Primer, D. N.; Molander, G. A . Science 2014, 345, 433.
doi: 10.1002/anie.201504963 |
|
|
(c) Corcé, V.; Chamoreau, L.-M.; Derat, E.; Goddard, J.-P.; Ollivier, C.; Fensterbank, L . Angew. Chem., Int. Ed. 2015, 54, 11414.
doi: 10.1002/anie.201504963 |
|
|
(d) Nakajima, K.; Nojima, S.; Nishibayashi, Y . Angew. Chem., Int. Ed. 2016, 55, 14106.
doi: 10.1002/anie.201504963 |
|
|
(e) Shaw, M. H.; Shurtleff, V. W.; Terrett, J. A.; Cuthbertson, J. D.; MacMillan, D. W. C . Science. 2016, 352, 1304.
doi: 10.1002/anie.201504963 |
|
|
(f) Heitz, D. R.; Tellis, J. C.; Molander, G. A . J. Am. Chem. Soc. 2016, 138, 12715.
doi: 10.1002/anie.201504963 |
|
| [17] |
For selected examples on copper metallaphotoredox catalysis, see: (a) Ye, Y.; Sanford, M. S. J. Am. Chem. Soc. 2012, 134, 9034.
doi: 10.1021/ja301553c |
|
(b) Yoo, W.-J.; Tsukamoto, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2015, 54, 6587.
doi: 10.1021/ja301553c |
|
| [18] |
For selected examples on gold metallaphotoredox catalysis, see: (a) Sahoo, B.; Hopkinson, M. N.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 5505.
doi: 10.1021/ja400311h |
|
(b) Shu, X.-Z.; Zhang, M.; He, Y.; Frei, H.; Toste, F. D. J. Am. Chem. Soc. 2014, 136, 5844.
doi: 10.1021/ja400311h |
|
| [19] |
(a) Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035.
doi: 10.1021/acs.chemrev.6b00018 |
|
(b) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A . Acc. Chem. Res. 2016, 49, 1429..
doi: 10.1021/acs.chemrev.6b00018 |
|
|
(c) Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C . Nature Rev. 2017, 1, 0052.
doi: 10.1021/acs.chemrev.6b00018 |
|
|
(d) Wang, C.-S.; Dixneuf, P. H.; Soulé, J.-F . Chem. Rev. 2018, 118, 7532.
doi: 10.1021/acs.chemrev.6b00018 |
|
|
(e) Zhou, W.-J.; Zhang, Y.-H.; Gui, Y.-Y.; Sun, L.; Yu, D.-G . Synthesis. 2018, 50, 3359.
doi: 10.1021/acs.chemrev.6b00018 |
|
|
(f) Chuentragool, P.; Kurandina, D.; Gevorgyan, V . Angew. Chem., Int. Ed. 2019, DOI: 10. 1002/anie. 201813523.
doi: 10.1021/acs.chemrev.6b00018 |
|
| [20] |
Lang, S. B.; O’Nele, K. M.; Tunge, J. A. J. Am. Chem. Soc. 2014, 136, 13606.
doi: 10.1021/ja4082793 |
| [21] |
Lang, S. B.; O’Nele, K. M.; Tunge, J. A. Chem. Eur. J. 2015, 21, 18589.
doi: 10.1002/chem.201503644 |
| [22] | Xuan, J.; Zeng, T.-T.; Feng,, Z,-J.; Deng, Q.-H.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J.; Alper, H. Angew. Chem., Int. Ed. 2015, 54, 1625. |
| [23] |
Jennifer, K. Matsui, J. K.; Gutiérrez-Bonet, A.; Rotella, M.; Alam, R.; Gutierrez, O.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57, 15847.
doi: 10.1002/anie.201809919 |
| [24] |
Thullen, S. M.; Rovis, T. J. Am. Chem. Soc. 2017, 139, 15504.
doi: 10.1021/jacs.7b09252 |
| [25] |
Zheng, J.; Breit, B. Angew. Chem., Int. Ed. 2019, 58, 3392.
doi: 10.1002/anie.201813646 |
| [26] |
Schwarz, J. L.; Schäfers, F.; Tlahuext-Aca, A.; Lückemeier, L.; Glorius, F. J. Am. Chem. Soc. 2018, 140, 12705.
doi: 10.1021/jacs.8b08052 |
| [27] |
For selected examples on asymmetric nickel metallaphotoredox catalysis, see:(a) Zuo, Z.; Cong, H.; Li, W.; Choi, J.; Fu, G. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 138, 1832.
doi: 10.1021/jacs.5b13211 |
|
(b) Stache, E. E.; Rovis, T.; Doyle, A. G . Angew. Chem., Int. Ed. 2017, 56, 3679. For selected examples on asymmetric copper metallaphotoredox catalysis, see:
doi: 10.1021/jacs.5b13211 |
|
|
(c) Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc. 2017, 139, 15632.
doi: 10.1021/jacs.5b13211 |
|
|
(d) Sha, W.; Deng, L.; Ni, S.; Mei, H.; Han, J.; Pan, Y . ACS Catal. 2018, 8, 7489.
doi: 10.1021/jacs.5b13211 |
|
| [28] |
Zhang, H.-H.; Zhao, J.-J.; Yu, S. J. Am. Chem. Soc. 2018, 140, 16914.
doi: 10.1021/jacs.8b10766 |
| [29] | Mitsunuma, H.; Tanabe, S.; Fuse, H.; Ohkubo, K.; Kanai, M . Chem. Sci. 2019, 10, 3459. |
| [1] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [2] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [3] | 韩明亮, 徐丽华. 过渡金属催化的硫酯的交叉偶联反应研究进展[J]. 化学学报, 2023, 81(4): 381-392. |
| [4] | 刘霞, 匡春香, 苏长会. 过渡金属催化的1,2,3-三氮唑导向的C—H键官能团化反应研究进展[J]. 化学学报, 2022, 80(8): 1135-1151. |
| [5] | 郭彩霞, 马小杰, 王博. 金属有机框架基复合材料的制备及其光热性能研究[J]. 化学学报, 2021, 79(8): 967-985. |
| [6] | 杨普苏, 刘晨旭, 张文文, 游书力. 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021, 79(6): 742-746. |
| [7] | 贾红绍, 乔保坤, 江智勇. 光氧化还原催化自由基偶联合成β-氟代-α-氨基酸衍生物[J]. 化学学报, 2021, 79(12): 1477-1480. |
| [8] | 杨启亮, 王向阳, 翁信军, 杨祥, 徐学涛, 童晓峰, 方萍, 伍新燕, 梅天胜. 电氧化促进的钯催化的芳烃C(sp 2)—H键氯代反应[J]. 化学学报, 2019, 77(9): 866-873. |
| [9] | 路福东, 姜烜, 陆良秋, 肖文精. 炔丙基自由基在有机合成化学中的应用[J]. 化学学报, 2019, 77(9): 803-813. |
| [10] | 王强, 顾庆, 游书力. 过渡金属催化不对称C—H键官能团化反应构建轴手性联芳基化合物研究进展[J]. 化学学报, 2019, 77(8): 690-704. |
| [11] | Khan Ijaz, 李红芳, 吴学, 张勇健. 钯配合物与手性方酰胺协同催化的乙烯基碳酸乙烯酯与醛的不对称脱羧环加成反应[J]. 化学学报, 2018, 76(11): 874-877. |
| [12] | 王乐明, 王骞, 陈杰安, 黄湧. Lewis酸对氮杂环卡宾协同催化体系中反应途径的调控[J]. 化学学报, 2018, 76(11): 850-856. |
| [13] | 余思凡, 傅祥, 刘耿鑫, 邱晃, 胡文浩. 通过不对称多组分反应高效构建手性磺酰胺类化合物[J]. 化学学报, 2018, 76(11): 895-900. |
| [14] | 周锵, 陆平. 手性路易斯碱和过渡金属协同催化反应的进展[J]. 化学学报, 2018, 76(11): 825-830. |
| [15] | 鲁鸿, 刘金宇, 李红玉, 许鹏飞. 氮杂环卡宾与过渡金属共催化反应研究进展[J]. 化学学报, 2018, 76(11): 831-837. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||