化学学报 ›› 2022, Vol. 80 ›› Issue (4): 485-493.DOI: 10.6023/A21120600 上一篇 下一篇
研究论文
陈守潇a, 刘君珂a, 郑伟琛b, 魏国祯c, 周尧a, 李君涛a,*( )
)
投稿日期:2021-12-30
发布日期:2022-04-28
通讯作者:
李君涛
基金资助:
Shouxiao Chena, Junke Liua, Weichen Zhengb, Guozhen Weic, Yao Zhoua, Juntao Lia( )
)
Received:2021-12-30
Published:2022-04-28
Contact:
Juntao Li
Supported by:文章分享
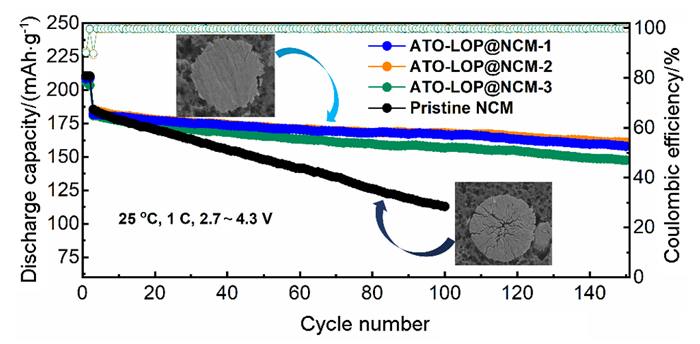
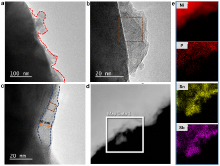
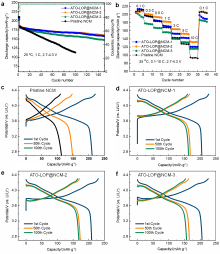
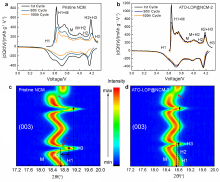
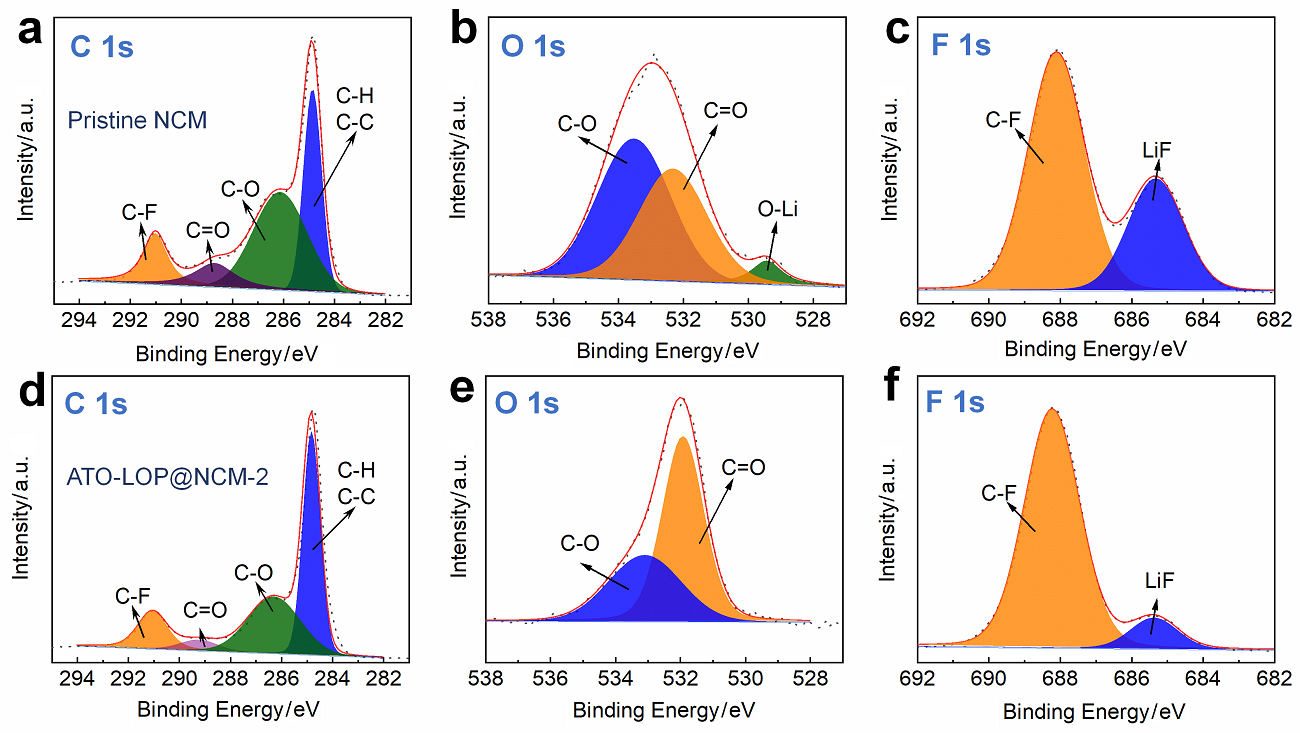
高镍三元材料LiNi0.8Co0.1Mn0.1O2 (NCM)比容量高且成本低, 但材料结构在电化学循环过程中的不稳定性影响了其大规模的应用, 可采用表面包覆的策略来改善材料的结构稳定性, 从而提高其电化学性能. 本工作结合高速固相包覆法和高温烧结法, 分别将电子导体氧化锡锑(ATO)和锂离子导体偏磷酸锂(LOP)共同包覆在NCM材料表面. 双包覆后的NCM材料的电子电导率从2.17×10-3 Ѕ•cm-1提高至1.02×10-2 Ѕ•cm-1, 锂离子扩散系数也从7.05×10-9 cm2•s-1提高至2.88×10-8 cm2•s-1. 同时, NCM表面的双包覆层可以在循环过程中阻止电极材料与电解液发生氧化还原反应, 抑制材料不利相变, 减少氧的析出, 稳定材料结构. 电化学性能测试表明, 经过表面包覆后, NCM材料在1 C (180 mA•g-1)的电流下和2.7~4.3 V (vs. Li/Li+)的电压范围内, 循环150周后容量为161.1 mAh•g-1, 保持率为87.1%, 而在10 C的充放电倍率下具有133 mAh•g-1的可逆比容量.
陈守潇, 刘君珂, 郑伟琛, 魏国祯, 周尧, 李君涛. 电/离子导体双包覆的LiNi0.8Co0.1Mn0.1O2锂离子电池阴极材料及其电化学性能[J]. 化学学报, 2022, 80(4): 485-493.
Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance[J]. Acta Chimica Sinica, 2022, 80(4): 485-493.








| [1] |
Chen, J. Acta Chim. Sinica 2017, 75, 127. (in Chinese)
doi: 10.6023/A1702E001 |
|
(陈军, 化学学报, 2017, 75, 127.)
doi: 10.6023/A1702E001 |
|
| [2] |
Goodenough, J. B.; Kim, Y. Chem. Mater. 2010, 22, 587.
doi: 10.1021/cm901452z |
| [3] |
Whittingham, M. S. Chem. Rev. 2004, 104, 4271.
pmid: 15669156 |
| [4] |
Zhou, H.; Xin, F.; Pei, B.; Whittingham, M. S. ACS Energy Lett. 2019, 4, 1902.
doi: 10.1021/acsenergylett.9b01236 |
| [5] |
Xu, P.; Zhang, X.-H.; Ma, E.; Rao, F.; Liu, C-W.; Yao, P.-F.; Sun, Z.; Wang, J.-W. Acta Chim. Sinica 2021, 79, 1073. (in Chinese)
doi: 10.6023/A21030083 |
|
(徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟, 化学学报, 2021, 79, 1073.)
doi: 10.6023/A21030083 |
|
| [6] |
Xia, L.; Yu, L.-P.; Hu, D.; Chen, Z. Acta Chim. Sinica 2017, 75, 1183. (in Chinese)
doi: 10.6023/A17060284 |
|
(夏兰, 余林颇, 胡笛, 陈政, 化学学报, 2017, 75, 1183.)
doi: 10.6023/A17060284 |
|
| [7] |
Yang, X.; Lin, M.; Zheng, G.; Wu, J.; Wang, X.; Ren, F.; Zhang, W.; Liao, Y.; Zhao, W.; Zhang, Z.; Xu, N.; Yang, W.; Yang, Y. Adv. Funct. Mater. 2020, 30, 2004664.
doi: 10.1002/adfm.202004664 |
| [8] |
Deng, B.-W.; Sun, D.-M.; Wan, Q.; Wang, H.; Chen, T.; Li, X.; Zhai, M. Z.; Peng, G.-C. Acta Chim. Sinica 2018, 76, 259. (in Chinese)
doi: 10.6023/A17110517 |
|
(邓邦为, 孙大明, 万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂, 化学学报, 2018, 76, 259.)
doi: 10.6023/A17110517 |
|
| [9] |
Qiu, K.; Yan, M.-X.; Zhao, S.-W.; An, S.-L.; Wang, W.; Jia, G.-X. Acta Chim. Sinica 2021, 79, 1146. (in Chinese)
doi: 10.6023/A21040178 |
|
(邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄, 化学学报, 2021, 79, 1146.)
doi: 10.6023/A21040178 |
|
| [10] |
Xin, F.; Zhou, H.; Zong, Y.; Zuba, M.; Chen, Y.; Chernova, N. A.; Bai, J.; Pei, B.; Goel, A.; Rana, J.; Wang, F.; An, K.; Piper, L. F. J.; Zhou, G.; Whittingham, M. S. ACS Energy Lett. 2021, 6, 1377.
|
| [11] |
Xiao, Y.; Miara, L. J.; Wang, Y.; Ceder, G. Joule 2019, 3, 1252.
doi: 10.1016/j.joule.2019.02.006 |
| [12] |
Lin, H.-C.; Yang, Y. Acta Chim. Sinica 2009, 67, 104. (in Chinese)
|
|
(林和成, 杨勇, 化学学报, 2009, 67, 104.)
|
|
| [13] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78, 1426. (in Chinese)
doi: 10.6023/A20070330 |
|
(刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益, 化学学报, 2020, 78, 1426.)
doi: 10.6023/A20070330 |
|
| [14] |
Ren, X.-Q.; Li, D.-L.; Zhao, Z.-Z.; Chen, G.-Q.; Zhao, K.; Kong, X.-Z.; Li, T.-X. Acta Chim. Sinica 2020, 78, 1268. (in Chinese)
doi: 10.6023/A20070319 |
|
(任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心, 化学学报, 2020, 78, 1268)
doi: 10.6023/A20070319 |
|
| [15] |
Liu, H.-H.; Zhang, J.; Lou, Y.-W.; Xia, B.-J. Acta Chim. Sinica 2012, 70, 1055. (in Chinese)
doi: 10.6023/A1112222 |
|
(刘浩涵, 张建, 娄豫皖, 夏保佳, 化学学报, 2012, 70, 1055.)
doi: 10.6023/A1112222 |
|
| [16] |
Kong, J.-Z.; Ren, C.; Tai, G.-A.; Zhang, X.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F. J. Power Sources 2014, 266, 433.
doi: 10.1016/j.jpowsour.2014.05.027 |
| [17] |
Myung, S.-T.; Izumi, K.; Komaba, S.; Yashiro, H.; Bang, H. J.; Sun, Y.-K.; Kumagai, N. J. Phys. Chem. C 2007, 111, 4061.
doi: 10.1021/jp0674367 |
| [18] |
Lu, Y.; Wang, J.-F.; Xie, H.-Q. Acta Chim. Sinica 2021, 79, 1058. (in Chinese)
doi: 10.6023/A21050213 |
|
(陆远, 王继芬, 谢华清, 化学学报, 2021, 79, 1058.)
doi: 10.6023/A21050213 |
|
| [19] |
Li, T.-X.; Li, D.-L.; Zhang, Q.-B.; Gao, J.-H.; Kong, X.-Z.; Fan, X.-Y.; Gou, L. Acta Chim. Sinica 2021, 79, 678. (in Chinese)
doi: 10.6023/A21010019 |
|
(李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾, 化学学报, 2021, 79, 678.)
doi: 10.6023/A21010019 |
|
| [20] |
Chang, Z.; Qiao, Y.; Yang, H.-Z.; Deng, H.; Zhu, X.-Y.; He, P.; Zhou, H.-S. Acta Chim. Sinica 2021, 79, 139. (in Chinese)
doi: 10.6023/A20090442 |
|
(常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎, 化学学报, 2021, 79, 139.)
doi: 10.6023/A20090442 |
|
| [21] |
Liang, Q.-M.; Guo, Y.-J.; Guo, J.-M.; Xiang, M.-W.; Liu, X.-F.; Bai, W.; Ning, P. Acta Chim. Sinica 2021, 79, 1526. (in Chinese)
doi: 10.6023/A21070324 |
|
(梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平, 化学学报, 2021, 79, 1526.)
doi: 10.6023/A21070324 |
|
| [22] |
Liu, J.; Xu, X.; Hu, R.; Yang, L.; Zhu, M. Adv. Energy Mater. 2016, 6, 1600256.
doi: 10.1002/aenm.201600256 |
| [23] |
Xu, Q.; Li, X.; Sari, H. M. K.; Li, W.; Liu, W.; Hao, Y.; Qin, J.; Cao, B.; Xiao, W.; Xu, Y.; Wei, Y.; Kou, L.; Tian, Z.; Shao, L.; Zhang, C.; Sun, X. Nano Energy, 2020, 77, 105034.
doi: 10.1016/j.nanoen.2020.105034 |
| [24] |
Wang, C.-W.; Zhou, Y.; You, J.-H.; Chen, J.-D.; Zhang, Z.; Zhang, S.-J.; Shi, C.-G.; Zhang, W.-D.; Zou, M.-H.; Yu, Y.; Li, J.-T.; Zeng, L.-Y.; Huang, L.; Sun, S.-G. ACS Appl. Energy Mater. 2020, 3, 2593.
doi: 10.1021/acsaem.9b02291 |
| [25] |
Griffith, K. J.; Wiaderek, K. M.; Cibin, G.; Marbella, L. E.; Grey, C. P. Nature 2018, 559, 556.
doi: 10.1038/s41586-018-0347-0 |
| [26] |
Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M.; Lin, R.; Chen, Y.; Li, Y.; Xing, X.; Choi, Y.; Gao, L.; Cho, H. S.; An, K.; Feng, J.; Kostecki, R.; Amine, K.; Wu, T.; Lu, J.; Xin, H. L.; Ong, S. P.; Liu, P. Nature 2020, 585, 63.
doi: 10.1038/s41586-020-2637-6 |
| [27] |
Guo, B.; Yu, X.; Sun, X.-G.; Chi, M.; Qiao, Z.-A.; Liu, J.; Hu, Y.-S.; Yang, X.-Q.; Goodenoughe, J. B.; Dai, S. Energy Environ. Sci. 2014, 7, 2220.
doi: 10.1039/C4EE00508B |
| [28] |
Ryu, H.-H.; Park, K.-J.; Yoon, D. R.; Aishova, A.; Yoon, C. S.; Sun, Y.-K. Adv. Energy Mater. 2019, 9, 1902698.
doi: 10.1002/aenm.201902698 |
| [29] |
Zhou, A.; Dai, X.; Lu, Y.; Wang, Q.; Fu, M.; Li, J. ACS Appl. Mater. Interfaces 2016, 8, 34123.
doi: 10.1021/acsami.6b11630 |
| [30] |
Zhang, X.; Zhang, X.; Sun, X.; An, Y.; Song, S.; Li, C.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. J. Power Sources 2021, 488, 229454.
doi: 10.1016/j.jpowsour.2021.229454 |
| [31] |
Li, J.; Manthiram, A. Adv. Energy Mater. 2019, 9, 1902731.
doi: 10.1002/aenm.201902731 |
| [32] |
Zou, L.; Zhao, W.; Liu, Z.; Jia, H.; Zheng, J.; Wang, G.; Yang, Y.; Zhang, J.-G.; Wang, C. ACS Energy Lett. 2018, 3, 2433.
doi: 10.1021/acsenergylett.8b01490 |
| [33] |
Das, H.; Urban, A.; Huang, W.; Ceder, G. Chem. Mater. 2017, 29, 7840.
doi: 10.1021/acs.chemmater.7b02546 |
| [34] |
Liu, W.; Oh, P.; Liu, X.; Lee, M. J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Angew. Chem. Int. Ed. 2015, 54, 4440.
doi: 10.1002/anie.201409262 |
| [35] |
Zhao, J.; Zhang, W.; Huq, A.; Misture, S. T.; Zhang, B.; Guo, S.; Wu, L.; Zhu, Y.; Chen, Z.; Amine, K.; Pan, F.; Bai, J.; Wang, F. Adv. Energy Mater. 2017, 7, 1601266.
doi: 10.1002/aenm.201601266 |
| [36] |
Kim, U. H.; Ryu, H. H.; Kim, J. H.; Mücke, R.; Kaghazchi, P.; Yoon, C. S.; Sun, Y. K. Adv. Energy Mater. 2019, 9, 1803902.
doi: 10.1002/aenm.201803902 |
| [1] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [2] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [3] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [4] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [5] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [6] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [7] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [8] | 徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081. |
| [9] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [10] | 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎. 金属有机框架(MOFs)材料在锂离子电池及锂金属电池电解液中的应用[J]. 化学学报, 2021, 79(2): 139-145. |
| [11] | 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益. 磷酸锂原位包覆富锂锰基锂离子电池正极材料[J]. 化学学报, 2020, 78(12): 1426-1433. |
| [12] | 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心. 铝掺杂及钨酸锂表面包覆双效提升富锂锰基正极材料的循环稳定性[J]. 化学学报, 2020, 78(11): 1268-1274. |
| [13] | 王珊, 樊小勇, 崔宇, 苟蕾, 王新刚, 李东林. 三维多孔集流体改善NiO电极的储锂特性[J]. 化学学报, 2019, 77(6): 551-558. |
| [14] | 王晓钰, 张渝, 马磊, 魏良明. 锂离子电池硅基负极粘结剂发展现状[J]. 化学学报, 2019, 77(1): 24-40. |
| [15] | 邓邦为, 孙大明, 万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂. 锂离子电池三元正极材料电解液添加剂的研究进展[J]. 化学学报, 2018, 76(4): 259-277. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||