化学学报 ›› 2022, Vol. 80 ›› Issue (4): 432-437.DOI: 10.6023/A21120597 上一篇 下一篇
所属专题: 中国科学院青年创新促进会合辑
研究通讯
投稿日期:2021-12-29
发布日期:2022-04-28
通讯作者:
邱早早
作者简介:基金资助:
Yixiu Gea, Zaozao Qiua( ), Zuowei Xiea,b
), Zuowei Xiea,b
Received:2021-12-29
Published:2022-04-28
Contact:
Zaozao Qiu
About author:Supported by:文章分享
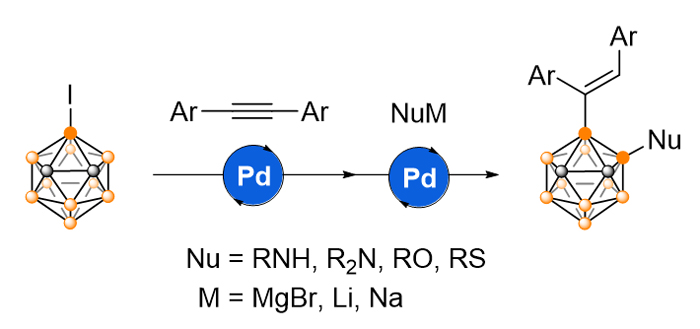
碳硼烷是由碳氢和硼氢顶点组成的笼状分子, 在医药、能源和材料等领域有着重要应用, 但目前在碳硼烷硼顶点引入杂原子取代基的方法还较为有限. 基于此, 本工作从3-碘-邻-碳硼烷出发, 通过钯催化烯基化、金属迁移及后续与杂原子亲核试剂的偶联反应, 一锅法构筑硼碳键和硼杂原子键, 成功实现了一系列新型3-烯基-4-胺基/烷氧基/烷(芳)硫基-邻-碳硼烷衍生物的合成.
葛懿修, 邱早早, 谢作伟. 钯催化硼-碳和硼-杂原子键构建一锅法合成双官能团化邻-碳硼烷※[J]. 化学学报, 2022, 80(4): 432-437.
Yixiu Ge, Zaozao Qiu, Zuowei Xie. Pd-Catalyzed One-Pot Synthesis of Difunctionalized o-Carboranes via Construction of B—C and B—Heteroatom Bonds※[J]. Acta Chimica Sinica, 2022, 80(4): 432-437.
| Entry | R | [M] | equiv. of 3 | Yieldb/% of 4 | |
|---|---|---|---|---|---|
| 1 | H | Li | 1.5 | 49 | |
| 2 | H | Li | 4.0 | —c | |
| 3 | H | Na | 1.5 | 44 | |
| 4 | H | MgBr | 1.5 | 87 (74)d | |
| 5 | H | MgBr | 1.2 | 75 | |
| 6 | H | MgBr | 2.0 | 73 | |
| 7 | H | MgBr | 3.0 | 69 | |
| 8 | Ph | Li | 1.5 | 55 | |
| 9e | Ph | Li | 1.5 | 60 (47)d | |
| 10 | Ph | MgBr | 1.5 | N.R. | |
| Entry | R | [M] | equiv. of 3 | Yieldb/% of 4 | |
|---|---|---|---|---|---|
| 1 | H | Li | 1.5 | 49 | |
| 2 | H | Li | 4.0 | —c | |
| 3 | H | Na | 1.5 | 44 | |
| 4 | H | MgBr | 1.5 | 87 (74)d | |
| 5 | H | MgBr | 1.2 | 75 | |
| 6 | H | MgBr | 2.0 | 73 | |
| 7 | H | MgBr | 3.0 | 69 | |
| 8 | Ph | Li | 1.5 | 55 | |
| 9e | Ph | Li | 1.5 | 60 (47)d | |
| 10 | Ph | MgBr | 1.5 | N.R. | |
| Entry | R1 | R2 | 4 | Yieldb/% |
|---|---|---|---|---|
| 1 | Ph | H | 4a | 74 |
| 2c | o-Me-C6H4 | H | 4b | 58 |
| 3 | m-Me-C6H4 | H | 4c | 65 |
| 4 | p-Me-C6H4 | H | 4d | 82 |
| 5c,d | p-MeO-C6H4 | H | 4e | 61 |
| 6 | p-F-C6H4 | H | 4f | 75 |
| 7 | p-CF3-C6H4 | H | — | N.R. |
| 8 | 2-Nap | H | 4g | 83 |
| 9 | 4-Py | H | — | N.R. |
| 10 | tBu | H | — | Messy |
| 11 | Bn | H | — | Messy |
| 12 | Ph | Ph | 4h | 47 |
| 13 | p-MeO-C6H4 | p-MeO-C6H4 | 4i | 51 |
| 14 | Ph | p-F-C6H4 | 4j | 60 |
| Entry | R1 | R2 | 4 | Yieldb/% |
|---|---|---|---|---|
| 1 | Ph | H | 4a | 74 |
| 2c | o-Me-C6H4 | H | 4b | 58 |
| 3 | m-Me-C6H4 | H | 4c | 65 |
| 4 | p-Me-C6H4 | H | 4d | 82 |
| 5c,d | p-MeO-C6H4 | H | 4e | 61 |
| 6 | p-F-C6H4 | H | 4f | 75 |
| 7 | p-CF3-C6H4 | H | — | N.R. |
| 8 | 2-Nap | H | 4g | 83 |
| 9 | 4-Py | H | — | N.R. |
| 10 | tBu | H | — | Messy |
| 11 | Bn | H | — | Messy |
| 12 | Ph | Ph | 4h | 47 |
| 13 | p-MeO-C6H4 | p-MeO-C6H4 | 4i | 51 |
| 14 | Ph | p-F-C6H4 | 4j | 60 |
| Entry | RO[M] | 6 | Yieldb/% |
|---|---|---|---|
| 1 | PhONa | — | N.R. |
| 2 | PhOMgBr | — | N.R. |
| 3 | MeONa | — | —c |
| 4d | tBuONa | 6a | 51 |
| Entry | RO[M] | 6 | Yieldb/% |
|---|---|---|---|
| 1 | PhONa | — | N.R. |
| 2 | PhOMgBr | — | N.R. |
| 3 | MeONa | — | —c |
| 4d | tBuONa | 6a | 51 |
| Entry | R | 8 | Yieldb/% |
|---|---|---|---|
| 1c | Me | 8a | 99 |
| 2c | Et | 8b | 81 |
| 3c,d | iPr | 8c | 77 |
| 4 | Ph | 8d | 99 |
| 5 | p-MeO-C6H4 | 8e | 62 |
| 6 | p-F-C6H4 | 8f | 66 |
| 7 | p-CF3-C6H4 | — | N.R. |
| Entry | R | 8 | Yieldb/% |
|---|---|---|---|
| 1c | Me | 8a | 99 |
| 2c | Et | 8b | 81 |
| 3c,d | iPr | 8c | 77 |
| 4 | Ph | 8d | 99 |
| 5 | p-MeO-C6H4 | 8e | 62 |
| 6 | p-F-C6H4 | 8f | 66 |
| 7 | p-CF3-C6H4 | — | N.R. |
| [1] |
Carboranes, 3rd ed., Ed.: Grimes, R. N., Elsevier, Oxford, U. K., 2016.
|
| [2] |
For selected reviews in applications in medicine, see: (a) Hawthorne, M. F.; Maderna, A. Chem. Rev. 1999, 99, 3421.
doi: 10.1021/cr980442h pmid: 31214680 |
|
(b) Scholz, M.; Hey-Hawkins, E. Chem. Rev. 2011, 111, 7035.
doi: 10.1021/cr200038x pmid: 31214680 |
|
|
(c) Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M. B.; Hey-Hawkins, E. Chem. Soc. Rev. 2019, 48, 3497.
doi: 10.1039/c9cs00197b pmid: 31214680 |
|
| [3] |
(a) Yang, X.; Jiang, W.; Knobler, C. B.; Hawthorne, M. F. J. Am. Chem. Soc. 1992, 114, 9719.
doi: 10.1021/ja00050a095 pmid: 30707011 |
|
(b) Jude, H.; Disteldorf, H.; Fischer, S.; Wedge, T.; Hawkridge, A. M.; Arif, A. M.; Hawthorne, M. F.; Muddiman, D. C.; Stang, P. J. J. Am. Chem. Soc. 2005, 127, 12131.
doi: 10.1021/ja053050i pmid: 30707011 |
|
|
(c) Koshino, M.; Tanaka, T.; Solin, N.; Suenaga, K.; Isobe, H.; Nakamura, E. Science 2007, 316, 853.
doi: 10.1126/science.1138690 pmid: 30707011 |
|
|
(d) Dash, B. P.; Satapathy, R.; Gaillard, E. R.; Maguire, J. A.; Hosmane, N. S. J. Am. Chem. Soc. 2010, 132, 6578.
doi: 10.1021/ja101845m pmid: 30707011 |
|
|
(e) Bauduin, P.; Prevost, S.; Farràs, P.; Teixidor, F.; Diat, O.; Zemb, T. Angew. Chem., Int. Ed. 2011, 50, 5298.
doi: 10.1002/anie.201100410 pmid: 30707011 |
|
|
(f) Cioran, A. M.; Musteti, A. D.; Teixidor, F.; Krpetić, Ž.; Prior, I. A.; He, Q.; Kiely, C. J.; Brust, M.; Viñas, C. J. Am. Chem. Soc. 2012, 134, 212.
doi: 10.1021/ja203367h pmid: 30707011 |
|
|
(g) Brusselle, D.; Bauduin, P.; Girard, L.; Zaulet, A.; Viñas, C.; Teixidor, F.; Ly, I.; Diat, O. Angew. Chem., Int. Ed. 2013, 52, 12114.
doi: 10.1002/anie.201307357 pmid: 30707011 |
|
|
(h) Schwartz, J. J.; Mendoza, M. A.; Wattanatorn, N.; Zhao, Y.; Nguyen, V. T.; Spokoyny, A. M.; Mirkin, C. A.; Baše, T.; Weiss, P. S. J. Am. Chem. Soc. 2016, 138, 5957.
doi: 10.1021/jacs.6b02026 pmid: 30707011 |
|
|
(i) Fisher, S. P.; Tomich, A. W.; Lovera, S. O.; Kleinsasser, J. F.; Guo, J.; Asay, M. J.; Nelson, H. M.; Lavallo, V. Chem. Rev. 2019, 119, 8262.
doi: 10.1021/acs.chemrev.8b00551 pmid: 30707011 |
|
|
(j) Cui, P.-F.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. J. Am. Chem. Soc. 2020, 142, 8532.
doi: 10.1021/jacs.0c03176 pmid: 30707011 |
|
|
(k) Cui, P.-F.; Liu, X.-R.; Guo, S.-T.; Lin, Y.-J.; Jin, G.-X. J. Am. Chem. Soc. 2021, 143, 5099.
doi: 10.1021/jacs.1c00779 pmid: 30707011 |
|
| [4] |
(a) Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F. F.; Teixidor, F. Chem. Rev. 2016, 116, 14307.
doi: 10.1021/acs.chemrev.6b00198 pmid: 30707011 |
|
(b) Fisher, S. P.; Tomich, A. W.; Lovera, S. O.; Kleinsasser, J. F.; Guo, J.; Asay, M. J.; Nelson, H. M.; Lavallo, V. Chem. Rev. 2019, 119, 8262.
doi: 10.1021/acs.chemrev.8b00551 pmid: 30707011 |
|
|
(c) Keener, M.; Hunt, C.; Carroll, T. G.; Kampel, V.; Dobrovetsky, R.; Hayton, T. W.; Ménard, G. Nature 2020, 577, 652.
doi: 10.1038/s41586-019-1926-4 pmid: 30707011 |
|
|
(d) Jung, D.; Saleh, L. M. A.; Berkson, Z. J.; El-Kady, M. F.; Hwang, J. Y.; Mohamed, N.; Wixtrom, A. I.; Titarenko, E.; Shao, Y.; McCarthy, K.; Guo, J.; Martini, I. B.; Kraemer, S.; Wegener, E. C.; Saint-Cricq, P.; Ruehle, B.; Langeslay, R. R.; Delferro, M.; Brosmer, J. L.; Hendon, C. H.; Gallagher-Jones, M.; Rodriguez, J.; Chapman, K. W.; Miller, J. T.; Duan, X.; Kaner, R. B.; Zink, J. I.; Chmelka, B. F.; Spokoyny, A. M. Nat. Mater. 2018, 17, 341.
doi: 10.1038/s41563-018-0021-9 pmid: 30707011 |
|
|
(e) Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Chem. Soc. Rev. 2016, 45, 5147.
doi: 10.1039/C6CS00159A pmid: 30707011 |
|
|
(f) Mukherjee, S.; Thilagar, P. Chem. Commun. 2016, 52, 1070.
doi: 10.1039/C5CC08213G pmid: 30707011 |
|
|
(g) Wei, X.; Zhu, M.-J.; Cheng, Z.; Lee, M.; Yan, H.; Lu, C.; Xu, J.-J. Angew. Chem., Int. Ed. 2019, 58, 3162.
doi: 10.1002/anie.201900283 pmid: 30707011 |
|
| [5] |
For selected reviews in applications in organometallic/coordination chemistry, see: (a) Xie, Z.. Coord. Chem. Rev. 2002, 231, 23.
doi: 10.1016/S0010-8545(02)00112-1 |
|
(b) Yao, Z.-J.; Jin, G.-X. Coord. Chem. Rev. 2013, 257, 2522.
doi: 10.1016/j.ccr.2013.02.004 |
|
|
(c) Fisher, S. P.; Tomich, A. W.; Guo, J.; Lavallo, V. Chem. Commun. 2019, 55, 1684.
doi: 10.1039/C8CC09663E |
|
| [6] |
(a) Grimes, R. N. Dalton Trans. 2015, 44, 5939.
doi: 10.1039/C5DT00231A |
|
(b) Olid, D.; Núñez, R.; Viñas, C.; Teixidor, F. Chem. Soc. Rev. 2013, 42, 3318.
doi: 10.1039/c2cs35441a |
|
|
(c) Dziedzic, R. M.; Spokoyny, A. M. Chem. Commun. 2019, 55, 430.
doi: 10.1039/C8CC08693A |
|
|
(d) Qiu, Z.; Ren, S.; Xie, Z. Acc. Chem. Res. 2011, 44, 299.
doi: 10.1021/ar100156f |
|
|
(e) Zhao, D.; Xie, Z. Coord. Chem. Rev. 2016, 314, 14.
doi: 10.1016/j.ccr.2015.07.011 |
|
|
(f) Zhang, X.; Yan, H. Coord. Chem. Rev. 2019, 378, 466.
doi: 10.1016/j.ccr.2017.11.006 |
|
|
(g) Mu, W.; Cheng, R.; Shang, Y.; He, R.; Li, D.; Fu, M. Chin. J. Org. Chem. 2018, 38, 1327. (in Chinese)
doi: 10.6023/cjoc201712044 |
|
|
(母伟花, 程瑞姣, 尚英伟, 贺仁泽, 李冬丽, 傅冕, 有机化学, 2018, 38, 1327.)
doi: 10.6023/cjoc201712044 |
|
|
(h) Xu, X.; Cheng, R.; Qiu, Z.; Pan, C. Chin. J. Org. Chem. 2018, 38, 3078. (in Chinese)
doi: 10.6023/cjoc201805018 |
|
|
(许新彬, 程若飞, 邱早早, 潘成岭, 2018, 38, 3078.)
|
|
|
(i) Mu, W.; Ma, Y.; Fang, D.; Wang, R.; Zhang, H. Acta Chim. Sinica 2018, 76, 55. (in Chinese)
doi: 10.6023/A17080357 |
|
|
(母伟花, 马瑶, 方德彩, 王蓉, 张海娜, 化学学报, 2018, 76, 55.)
doi: 10.6023/A17080357 |
|
| [7] |
(a) Zakharkin, L. I.; Stanko, V. I.; Brattstev, V. A.; Chapovskii, Y. A.; Struchkov, Y. T. Russ. Chem. Bull. 1963, 12, 1911.
doi: 10.1007/BF00843831 |
|
(b) Zakharkin, L. I.; Stanko, V. I.; Brattsev, V. A.; Chapovskii, Y. A.; Okhlobystin, O. Y. Russ. Chem. Bull. 1963, 12, 2074.
doi: 10.1007/BF00844026 |
|
|
(c) Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. Inorg. Chem. 1963, 2, 1089.
doi: 10.1021/ic50010a002 |
|
|
(d) Fein, M. M.; Bobinski, J.; Mayes, N.; Schwartz, N.; Cohen, M. S. Inorg. Chem. 1963, 2, 1111.
doi: 10.1021/ic50010a007 |
|
| [8] |
(a) Quan, Y.; Qiu, Z.; Xie, Z. Chem. Eur. J. 2018, 24, 2795.
doi: 10.1002/chem.201704937 |
|
(b) Quan, Y.; Xie, Z. Chem. Soc. Rev. 2019, 48, 3660.
doi: 10.1039/C9CS00169G |
|
|
(c) Au, Y. K.; Xie, Z. Bull. Chem. Soc. Jpn. 2021, 94, 879.
doi: 10.1246/bcsj.20200366 |
|
|
(d) Qiu, Z.; Xie, Z. Acc. Chem. Res. 2021, 54, 4065.
doi: 10.1021/acs.accounts.1c00460 |
|
|
(e) Lian, L.; Yin, J.; Lin, C.; Ye, K.; Yuan, Y. Chin. J. Org. Chem. 2021, 41, 3249. (in Chinese)
doi: 10.6023/cjoc202103015 |
|
|
(连凌翔, 尹静怡, 林彩霞, 叶克印, 袁耀锋, 有机化学, 2021, 41, 3249.)
doi: 10.6023/cjoc202103015 |
|
|
(f) Zhang, H.; Qiu, Z.; Xie, Z. Chin. J. Org. Chem. 2020, 40, 3203. (in Chinese)
doi: 10.6023/cjoc202005079 |
|
|
(张慧芳, 邱早早, 谢作伟, 有机化学, 2020, 40, 3203.)
doi: 10.6023/cjoc202005079 |
|
|
(g) Liu, X.-R.; Cui, P.-F.; Guo, S.-T.; Yuan, R.-Z.; Jin, G.-X. Inorg. Chem. Front. 2021, 8, 4349.
doi: 10.1039/D1QI00732G |
|
| [9] |
Zhang, Z.-Y.; Zhang, X.; Yuan, J.; Yue, C.-D.; Meng, S.; Chen, J.; Yu, G.-A.; Che, C.-M. Chem. Eur. J. 2020, 26, 5037.
doi: 10.1002/chem.201905647 |
| [10] |
Cheng, R.; Qiu, Z.; Xie, Z. Nat. Commun. 2017, 8, 14827.
doi: 10.1038/ncomms14827 |
| [11] |
(a) Lyu, H.; Quan, Y.; Xie, Z. Angew. Chem., Int. Ed. 2016, 55, 11840.
doi: 10.1002/anie.201605880 |
|
(b) Guo, S.-T.; Cui, P.-F.; Yuan, R.-Z.; Jin, G.-X. Chem. Commun. 2021, 57, 2412.
doi: 10.1039/D0CC08290B |
|
| [12] |
(a) Li, C.-X.; Zhang, H.-Y.; Wong, T.-Y.; Cao, H.-J.; Yan, H.; Lu, C.-S. Org. Lett. 2017, 19, 5178.
doi: 10.1021/acs.orglett.7b02450 |
|
(b) Cao, K.; Xu, T.-T.; Wu, J.; Jiang, L.; Yang, J. Chem. Commun. 2016, 52, 11446.
doi: 10.1039/C6CC06200H |
|
| [13] |
(a) Dziedzic, R. M.; Martin, J. L.; Axtell, J. C.; Saleh, L. M. A.; Ong, T.-C.; Yang, Y.-F.; Messina, M. S.; Rheingold, A. L.; Houk, K. N.; Spokoyny, A. M. J. Am. Chem. Soc. 2017, 139, 7729.
doi: 10.1021/jacs.7b04080 pmid: 28541671 |
|
(b) Cui, C. X.; Zhang, J.; Qiu, Z.; Xie, Z. Dalton Trans. 2020, 49, 1380.
doi: 10.1039/C9DT04553H pmid: 28541671 |
|
|
(c) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2020, 142, 6940.
doi: 10.1021/jacs.0c02490 pmid: 28541671 |
|
| [14] |
(a) Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2016, 138, 12727.
doi: 10.1021/jacs.6b07086 |
|
(b) Au, Y. K.; Zhang, J.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2021, 143, 4148.
doi: 10.1021/jacs.1c00593 |
|
| [15] |
(a) Han, G. U.; Baek, Y.; Lee, K.; Shin, S.; Chan Noh, H.; Lee, P. H. Org. Lett. 2021, 23, 416.
doi: 10.1021/acs.orglett.0c03927 pmid: 33377789 |
|
(b) Baek, Y.; Kim, S.; Son, J.-Y.; Lee, K.; Kim, D.; Lee, P. H. ACS Catal. 2019, 9, 10418.
doi: 10.1021/acscatal.9b03380 pmid: 33377789 |
|
| [16] |
Chen, Y.; Quan, Y.; Xie, Z. Chem. Commun. 2020, 56, 12997.
doi: 10.1039/D0CC05207H |
| [17] |
(a) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. Chin. J. Chem. 2020, 38, 383.
doi: 10.1002/cjoc.201900475 |
|
(b) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2019, 141, 12855.
doi: 10.1021/jacs.9b06204 |
|
|
(c) Cheng, B.; Chen, Y.; Zhou, P.; Xie, Z. Chem. Commun. 2022, DOI: 10.1039/D1CC05936J.
doi: 10.1039/D1CC05936J |
|
|
(d) Cao, H.-J.; Chen, M.; Sun, F.; Zhao, Y.; Lu, C.; Zhang, X.; Shi, Z.; Yan, H. ACS Catal. 2021, 11, 14047.
doi: 10.1021/acscatal.1c04473 |
|
| [18] |
(a) Li, J.; Logan, C. F.; Jones, M. Inorg. Chem. 1991, 30, 4866.
doi: 10.1021/ic00025a037 pmid: 11735463 |
|
(b) Viñas, C.; Barberà, G.; Oliva, J. M.; Teixidor, F.; Welch, A. J.; Rosair, G. M. Inorg. Chem. 2001, 40, 6555.
pmid: 11735463 |
|
| [19] |
Sevryugina, Y.; Julius, R.; Hawthorne, M. F. Inorg. Chem. 2010, 49, 10627.
doi: 10.1021/ic101620h pmid: 20964311 |
| [20] |
Dziedzic, R. M.; Saleh, L. M. A.; Axtell, J. C.; Martin, J. L.; Stevens, S. L.; Timothy Royappa, A.; Rheingold, A. L.; Spokoyny, A. M. J. Am. Chem. Soc. 2016, 138, 9081.
doi: 10.1021/jacs.6b05505 pmid: 27384544 |
| [21] |
Kabytaev, K. Z.; Everett, T. A.; Safronov, A. V.; Sevryugina, Y. V.; Jalisatgi, S. S.; Hawthorne, M. F. Eur. J. Inorg. Chem. 2013, 2488.
|
| [22] |
(a) Liu, D.; Dang, L.; Sun, Y.; Chan, H.-S.; Lin, Z.; Xie, Z. J. Am. Chem. Soc. 2008, 130, 16103.
doi: 10.1021/ja8067098 pmid: 28541671 |
|
(b) Eleazer, B. J.; Smith, M. D.; Popov, A. A.; Peryshkov, D. V. Chem. Sci. 2017, 8, 5399.
doi: 10.1039/C7SC01846K pmid: 28541671 |
|
|
(c) Dziedzic, R. M.; Axtell, J. C.; Rheingold, A. L.; Spokoyny, A. M. Org. Process Res. Dev. 2019, 23, 1638.
doi: 10.1021/acs.oprd.9b00257 pmid: 28541671 |
|
|
(d) Dziedzic, R. M.; Martin, J. L.; Axtell, J. C.; Saleh, L. M. A.; Ong, T.-C.; Yang, Y.-F.; Messina, M. S.; Rheingold, A. L.; Houk, K. N.; Spokoyny, A. M. J. Am. Chem. Soc. 2017, 139, 7729.
doi: 10.1021/jacs.7b04080 pmid: 28541671 |
|
|
(e) Lyu, H.; Zhang, J.; Yang, J.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2019, 141, 4219.
doi: 10.1021/jacs.9b00302 pmid: 28541671 |
|
|
(f) Guo, C.; Qiu, Z.; Xie, Z. ACS Catal. 2021, 11, 2134.
doi: 10.1021/acscatal.0c05639 pmid: 28541671 |
|
| [23] |
(a) Ge, Y.; Zhang, J.; Qiu, Z.; Xie, Z. Angew. Chem., Int. Ed. 2020, 59, 4851.
doi: 10.1002/anie.201914500 |
|
(b) Ge, Y.; Qiu, Z.; Xie, Z. Chem. Commun. 2021, 57, 8071.
doi: 10.1039/D1CC03449A |
|
|
(c) Ge, Y.; Zhang, J.; Qiu, Z.; Xie, Z. Dalton Trans. 2021, 50, 1766.
doi: 10.1039/D0DT03740K |
| [1] | 张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚. 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024, 82(2): 105-109. |
| [2] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [3] | 徐云芳, 李阳, 付梓桐, 林绍艳, 祝洁, 吴磊. 钯催化选择性构筑(Z)-[3]戟烯反应研究[J]. 化学学报, 2022, 80(10): 1369-1375. |
| [4] | 李忠原, 景昆, 李祁利, 王官武. 钯催化的2-苯氧基吡啶导向的脱羧酯基化反应研究[J]. 化学学报, 2019, 77(8): 729-734. |
| [5] | Khan Ijaz, 李红芳, 吴学, 张勇健. 钯配合物与手性方酰胺协同催化的乙烯基碳酸乙烯酯与醛的不对称脱羧环加成反应[J]. 化学学报, 2018, 76(11): 874-877. |
| [6] | 周霄乐, 苏永亮, 汪普生, 龚流柱. 1,4-二烯和醛的烯丙基碳氢不对称烷基化反应[J]. 化学学报, 2018, 76(11): 857-861. |
| [7] | 母伟花, 马瑶, 方德彩, 王蓉, 张海娜. 1-碘-2-锂-邻碳硼烷与环戊二烯衍生物的类Diels-Alder反应的理论研究[J]. 化学学报, 2018, 76(1): 55-61. |
| [8] | 张子競, 陶忠林, 阿拉法特·阿地力, 龚流柱. 钯配合物和手性磷酸连续催化的烯丙醇和醛的不对称羰基烯丙基化反应[J]. 化学学报, 2017, 75(12): 1196-1201. |
| [9] | 汤淏溟, 霍小红, 孟庆华, 张万斌. 钯催化的烯丙位C—H键官能团化:新催化体系的发展[J]. 化学学报, 2016, 74(3): 219-233. |
| [10] | 罗飞华, 龙洋, 李正凯, 周向葛. 水相钯(II)催化羰基β位C(sp3)-H芳基化反应[J]. 化学学报, 2016, 74(10): 805-810. |
| [11] | 徐佳斌, 陈品红, 叶金星, 刘国生. 钯催化的芳基C-H键三氟甲硫基化反应[J]. 化学学报, 2015, 73(12): 1294-1297. |
| [12] | 杜乐, 曹鹏, 廖建. 双功能配体促进的钯催化不对称醚化和胺化反应[J]. 化学学报, 2013, 71(9): 1239-1242. |
| [13] | 蔡海婷, 李丹丹, 刘姿, 王官武. 钯催化下肟醚导向的sp2 C—H键邻位酰氧化反应[J]. 化学学报, 2013, 71(05): 717-721. |
| [14] | 温燕梅, 江焕峰. 钯催化炔溴和烯烃偶联合成共轭烯炔的研究[J]. 化学学报, 2012, 70(16): 1716-1720. |
| [15] | 张斌彬, 詹丹, 张小平, 向沁洁, 曾庆乐. 空气下无配体钯催化二苯胺和卤代芳烃的C—N偶联[J]. 化学学报, 2012, 70(15): 1655-1659. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||