化学学报 ›› 2022, Vol. 80 ›› Issue (3): 277-281.DOI: 10.6023/A21120588 上一篇 下一篇
所属专题: 中国科学院青年创新促进会合辑
研究论文
王孟孟a, 张俊b, 王慧颖a, 马彪b,*( ), 戴辉雄a,b,c,*(
), 戴辉雄a,b,c,*( )
)
投稿日期:2021-12-27
发布日期:2022-01-30
通讯作者:
马彪, 戴辉雄
作者简介:基金资助:
Mengmeng Wanga, Jun Zhangb, Huiying Wanga, Biao Mab( ), Hui-Xiong Daia,b,c(
), Hui-Xiong Daia,b,c( )
)
Received:2021-12-27
Published:2022-01-30
Contact:
Biao Ma, Hui-Xiong Dai
About author:Supported by:文章分享
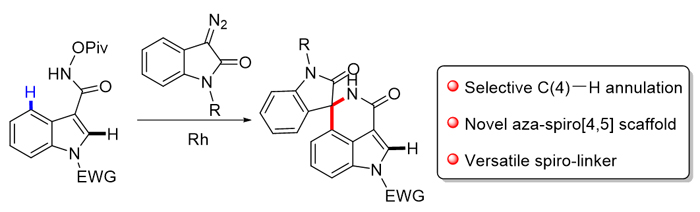
螺环吲哚是天然产物和药物化学中的一类重要骨架, 通过导向基团导向的C—H键活化反应已经成为构建螺环吲哚的重要方法. 目前在吲哚的吡咯片段上引入螺环结构已经比较成熟, 然而在吲哚的苯环片段上引入螺环还存在挑战. 以过渡金属铑催化, 选择性地活化吲哚C(4)—H键, 高效构建了氮杂-螺[4,5]吲哚骨架.
王孟孟, 张俊, 王慧颖, 马彪, 戴辉雄. 铑催化吲哚C(4)—H选择性活化构建氮杂-螺[4,5]吲哚骨架※[J]. 化学学报, 2022, 80(3): 277-281.
Mengmeng Wang, Jun Zhang, Huiying Wang, Biao Ma, Hui-Xiong Dai. Construction of Aza-spiro[4,5]indole Scaffolds via Rhodium-Catalyzed Regioselective C(4)—H Activation of Indole※[J]. Acta Chimica Sinica, 2022, 80(3): 277-281.
| Entry | Changes | Yieldb/% | |
|---|---|---|---|
| 1 | None | 87 (84c) | |
| 2 | MeOH as solvent | 36 | |
| 3 | THF as solvent | 50 | |
| 4 | KOAc as additive | 81 | |
| 5 | AgSbF6 as additive | 0 | |
| 6 | No NaOAc | 0 | |
| 7 | R=Me or Bn | 0 | |
| 8 | 60 ℃ | 69 | |
| 9 | 4 h | 75 |
| Entry | Changes | Yieldb/% | |
|---|---|---|---|
| 1 | None | 87 (84c) | |
| 2 | MeOH as solvent | 36 | |
| 3 | THF as solvent | 50 | |
| 4 | KOAc as additive | 81 | |
| 5 | AgSbF6 as additive | 0 | |
| 6 | No NaOAc | 0 | |
| 7 | R=Me or Bn | 0 | |
| 8 | 60 ℃ | 69 | |
| 9 | 4 h | 75 |
| [1] |
For selected reviews: (a) Chauhan, M.; Saxena, A.; Saha, B.. Eur. J. Med. Chem. 2021, 218, 113400.
doi: 10.1016/j.ejmech.2021.113400 pmid: 20380420 |
|
(b) Thanikachalam, P. V.; Maurya, R. K.; Garg, V.; Monga, V. Eur. J. Med. Chem. 2019, 180, 562.
doi: 10.1016/j.ejmech.2019.07.019 pmid: 20380420 |
|
|
(c) Sravanthi, T. V.; Manju, S. L. Eur. J. Pharm. Sci. 2016, 91, 1.
doi: 10.1016/j.ejps.2016.05.025 pmid: 20380420 |
|
|
(d) Somei, M.; Yamada, F. Nat. Prod. Rep. 2005, 22, 73.
doi: 10.1039/b316241a pmid: 20380420 |
|
|
(e) Kochanowska-Karamyan, A. J.; Hamann, M. T. Chem. Rev. 2010, 110, 4489.
doi: 10.1021/cr900211p pmid: 20380420 |
|
| [2] |
For selected examples: (a) Hiesinger, K.; Dar'in, D.; Proschak, E.; Krasavin, M. J. Med. Chem. 2021, 64, 150.
doi: 10.1021/acs.jmedchem.0c01473 pmid: 29521213 |
|
(b) Ghatpande, N. G.; Jadhav, J. S.; Kaproormath, R. V.; Soliman, M. E.; Shaikh, M. M. Bioorg. Med. Chem. 2020, 28, 115813.
doi: 10.1016/j.bmc.2020.115813 pmid: 29521213 |
|
|
(c) Ren, B.-Y.; Yi, J.-C.; Zhong, D.-K.; Zhao, Y.-Z.; Guo, R.-D.; Sheng, Y.-G.; Sun, Y.-G.; Xie, L.-H.; Huang, W. Acta Chim. Sinica 2020, 78, 56. (in Chinese)
doi: 10.6023/A19110406 pmid: 29521213 |
|
|
(任保轶, 依建成, 钟道昆, 赵玉志, 郭闰达, 盛永刚, 孙亚光, 解令海, 黄维, 化学学报, 2020, 78, 56.)
doi: 10.6023/A19110406 pmid: 29521213 |
|
|
(d) Benabdallaha, M.; Talhib, O.; Noualia, F.; Choukchou-Brahama, N.; Bacharib, K.; Silva, A. M. S. Curr. Med. Chem. 2018, 25, 3748.
doi: 10.2174/0929867325666180309124821 pmid: 29521213 |
|
| [3] |
For selected examples: (a) Nasri, S.; Bayat, M.; Mirzaei, F. Top. Curr. Chem. 2021, 379, 25.
|
|
(b) Bora, D.; Kaushal, A.; Shankaraiah, N. Eur. J Med. Chem. 2021, 215, 113263.
doi: 10.1016/j.ejmech.2021.113263 |
|
|
(c) Li, B.; Zhou, R.; He, G.; Guo, L.; Huang, W. Acta Chim. Sinica 2013, 71, 1396. (in Chinese)
doi: 10.6023/A13040375 |
|
|
(李博, 周锐, 何谷, 郭丽, 黄维, 化学学报, 2013, 71, 1396.)
doi: 10.6023/A13040375 |
|
| [4] |
For selected examples: (a) Zhou, B.; Liang, R.; Cao, Z.; Zhou, P.; Jia, Y. Acta Chim. Sinica 2021, 79, 176 (in chinses).
doi: 10.6023/A20110520 pmid: 28981293 |
|
(周波, 梁仁校, 曹中艳, 周平海, 贾义霞, 化学学报, 2021, 79, 176.)
doi: 10.6023/A20110520 pmid: 28981293 |
|
|
(b) Li, N.-K.; Zhang, J.-Q.; Sun, B.-B.; Li, H.-Y.; Wang, X.-W. Org. Lett. 2017, 19, 1954.
doi: 10.1021/acs.orglett.7b00368 pmid: 28981293 |
|
|
(c) Chen, X.-Y.; Baratay, C. A.; Mark, M. E.; Xu, X.-F.; Chan, P. W. H. Org. Lett. 2020, 22, 2849.
doi: 10.1021/acs.orglett.0c00929 pmid: 28981293 |
|
|
(d) Zhu, M.; Zheng, C.; Zhang, X.; You, S.-L. J. Am. Chem. Soc. 2019, 141, 2636.
doi: 10.1021/jacs.8b12965 pmid: 28981293 |
|
|
(e) Tang, S.; Wang, J.; Xiong, Z.; Xie, Z.; Li, D.; Huang, J.; Zhu, Q. Org. Lett. 2017, 19, 5577.
doi: 10.1021/acs.orglett.7b02725 pmid: 28981293 |
|
|
(f) Qiu, H.; Zhang, D.; Liu, S.; Qiu, L.; Zhou, J.; Qian, Y.; Zhai, C.; Hu, W. Acta Chim. Sinica. 2012, 70, 2484. (in Chinese)
doi: 10.6023/A12100821 pmid: 28981293 |
|
|
(邱晃, 张丹, 刘顺英, 邱林, 周俊, 钱宇, 翟昌伟, 胡文浩, 化学学报, 2012, 70, 2484.)
doi: 10.6023/A12100821 pmid: 28981293 |
|
| [5] |
For selected reviews: (a) Sinha, S. K.; Guin, S.; Maiti, S.; Biswas, J. P.; Porey, S.; Maiti, D. Chem. Rev. 2021, DOI: 10.1021/acs.chemrev.1c00220.
doi: 10.1021/acs.chemrev.1c00220 |
|
(b) Zhu, W.-H.; Gunnoe, T. B. Acc. Chem. Res. 2020, 53, 920.
doi: 10.1021/acs.accounts.0c00036 |
|
|
(c) Rej, S.; Chatani, N. Angew. Chem., Int. Ed. 2019, 58, 8304.
doi: 10.1002/anie.v58.25 |
|
|
(d) Hong, J.; Li, M.; Zhang, J.; Sun, B.; Mo, F. ChemSusChem 2019, 12, 6.
doi: 10.1002/cssc.v12.1 |
|
|
(e) Chu, J. C. K.; Rovis, T. Angew. Chem., Int. Ed. 2018, 57, 62.
doi: 10.1002/anie.201703743 |
|
|
(f) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754.
doi: 10.1021/acs.chemrev.6b00622 |
|
|
(g) Zhu, R.-Y.; Farmer, M. E.; Chen, Y.-Q.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 10578.
doi: 10.1002/anie.v55.36 |
|
|
(h) Zhao, J.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235. (in Chinese)
doi: 10.6023/A15010063 |
|
|
(赵金钵, 张前, 化学学报, 2015, 73, 1235.)
doi: 10.6023/A15010063 |
|
| [6] |
(a) Zeng, H.-Y.; Wang, Z.-M.; Li, C.-J. Angew. Chem., Int. Ed. 2019, 58, 2859.
doi: 10.1002/anie.v58.9 pmid: 24959967 |
|
(b) Wang, Z.-M.; Niu, J.-B.; Zeng, H.-Y.; Li, C.-J. Org. Lett. 2019, 21, 7033.
doi: 10.1021/acs.orglett.9b02613 pmid: 24959967 |
|
|
(c) Zheng, J.; Zhang, Y.; Cui, S.-L. Org. Lett. 2014, 16, 3560.
doi: 10.1021/ol5014312 pmid: 24959967 |
|
|
(d) Chabaud, L.; Raynal, Q.; Barre, E.; Guillou, C. Adv. Synth. Catal. 2015, 357, 3880.
doi: 10.1002/adsc.201500768 pmid: 24959967 |
|
| [7] |
Zhang, Y.; Zheng, J.; Cui, S.-L. J. Org. Chem. 2014, 79, 6490.
doi: 10.1021/jo500902n pmid: 24949803 |
| [8] |
Zhang, J.; Wang, M.; Wang, H.; Xu, H.; Chen, J.; Guo, Z.; Ma, B.; Ban, S.-R.; Dai, H.-X. Chem. Commun. 2021, 57, 8656.
doi: 10.1039/D1CC02798K |
| [9] |
(a) Kumar, P.; Nagtilak, P. J.; Kapur, M. New J. Chem. 2021, 45, 13692.
doi: 10.1039/D1NJ01696B |
|
(b) Kalepu, J.; Gandeepan, P.; Ackermann, L.; Pilarski, L. T. Chem. Sci. 2018, 9, 4203.
doi: 10.1039/C7SC05336C |
|
| [10] |
For selected reviews: (a) Wen, J.; Shi, Z. Acc. Chem. Res. 2021, 54, 1723.
doi: 10.1021/acs.accounts.0c00888 pmid: 34486584 |
|
(b) Prabagar, B.; Yang, Y.; Shi, Z. Chem. Soc. Rev. 2021, 50, 11249.
doi: 10.1039/d0cs00334d pmid: 34486584 |
|
| [11] |
For selected examples: (a) Kuang, G.; Liu, D.; Chen, X.; Liu, G.; Fu, Y.; Peng, Y.; Li, H.; Zhou, Y. Org. Lett. 2021, 23, 8402.
doi: 10.1021/acs.orglett.1c03131 |
|
(b) Lanke, V.; Prabhu, K. R. Chem. Commun. 2017, 53, 5117.
doi: 10.1039/C7CC00763A |
|
|
(c) Liu, X.; Li, G.; Song, F.; You, J. Nat. Commun. 2014, 5, 5030.
doi: 10.1038/ncomms6030 |
|
|
(d) Chen, S.; Feng, B.; Zheng, X.; Yin, J.; Yang, S.; You, J. Org. Lett. 2017, 19, 2502.
doi: 10.1021/acs.orglett.7b00730 |
|
| [12] |
For selected examples: (a) Cheng, Y.; Yu, S.; He, Y.; An, G.; Li, G.; Yang, Z. Chem. Sci. 2021, 12, 3216.
doi: 10.1039/D0SC05409G pmid: 31545620 |
|
(b) Banjare, S. K.; Nanda, T.; Pati, B. V.; Das Adhikari, G. K.; Dutta, J.; Ravikumar, P. C. ACS Catal. 2021, 11, 11579.
doi: 10.1021/acscatal.1c02689 pmid: 31545620 |
|
|
(c) Harada, S.; Yanagawa, M.; Nemoto, T. ACS Catal. 2020, 10, 11971.
doi: 10.1021/acscatal.0c03940 pmid: 31545620 |
|
|
(d) Pradhan, S.; Mishra, M.; Bhusan De, P.; Banerjee, S.; Punniyamurthy, T. Org. Lett. 2020, 22, 1720.
doi: 10.1021/acs.orglett.9b04612 pmid: 31545620 |
|
|
(e) Lv, J.; Chen, X.; Xu, X.-S.; Zhao, B.; Liang, Y.; Wang, M.; Jin, L.; Yuan, Y.; Han, Y.; Zhao, Y.; Lu, Y.; Zhao, J.; Sun, W.-Y.; Houk, K. N.; Shi, Z. Nature 2019, 575, 336.
doi: 10.1038/s41586-019-1640-2 pmid: 31545620 |
|
|
(f) Yang, Y.; Gao, P.; Zhao, Y.; Shi, Z. Angew. Chem., Int. Ed. 2017, 56, 3966.
doi: 10.1002/anie.201612599 pmid: 31545620 |
|
|
(g) Sherikar, M. S.; Kapanaiah, R.; Lanke, V.; Prabhu, K. R. Chem. Commun. 2018, 54, 11200.
doi: 10.1039/C8CC06264A pmid: 31545620 |
|
|
(h) Zhang, J.; Wu, M.; Fan, J.; Xu, Q.; Xie, M. Chem. Commun. 2019, 55, 8102.
doi: 10.1039/C9CC03893K pmid: 31545620 |
|
|
(i) Banjare, S. K.; Nanda, T.; Ravikumar, P. C. Org. Lett. 2019, 21, 8138.
doi: 10.1021/acs.orglett.9b03243 pmid: 31545620 |
|
| [13] |
Maity, S.; Karmakar, U.; Samanta, R. Chem. Commun. 2017, 53, 12197.
doi: 10.1039/C7CC07086A |
| [14] |
For selected examples: (a) Zhang, Q.; Xie, X.; Peng, J.; Chen, F.; Ma, J.; Li, C.; Liu, H.; Wang, D.; Wang, J. Org. Lett. 2021, 23, 4699.
doi: 10.1021/acs.orglett.1c01434 pmid: 34060854 |
|
(b) Bai, Z.; Cai, C.; Sheng, W.; Ren, Y.; Wang, H. Angew. Chem., Int. Ed. 2020, 59, 14686.
doi: 10.1002/anie.v59.34 pmid: 34060854 |
|
|
(c) Liu, Q.; Li, Q.; Ma, Y.; Jia, Y. Org. Lett. 2013, 15, 4528.
doi: 10.1021/ol4020877 pmid: 34060854 |
|
|
(d) Ge, Y.; Wang, H.; Wang, H.-N.; Yu, S.-S.; Yang, R.; Chen, X.; Zhao, Q.; Chen, G. Org. Lett. 2021, 23, 370.
doi: 10.1021/acs.orglett.0c03867 pmid: 34060854 |
|
| [15] |
For selected examples: (a) Kona, C. N.; Nishii, Y.; Miura, M. Angew. Chem., Int. Ed. 2019, 58, 9856.
doi: 10.1002/anie.v58.29 |
|
(b) Kona, C. N.; Nishii, Y.; Miura, M. Org. Lett. 2018, 20, 4898.
doi: 10.1021/acs.orglett.8b02038 |
| [1] | 江崇国, 陈斯嘉, 龚建贤, 杨震. Phainanoids的4,5-螺环骨架的合成探索[J]. 化学学报, 2020, 78(9): 928-932. |
| [2] | 鲁平, 冯超, 罗德平. Rh/Ag双金属催化的碳氢键氧化Heck反应研究[J]. 化学学报, 2015, 73(12): 1315-1319. |
| [3] | 李博, 周锐, 何谷, 郭丽, 黄维. 螺环吲哚类MDM2抑制剂的分子对接、定量构效关系和分子动力学模拟[J]. 化学学报, 2013, 71(10): 1396-1403. |
| [4] | 李明, 罗小玲, 唐典勇. 铑催化烯烃氢甲酰化反应的密度泛函研究[J]. 化学学报, 2004, 62(12): 1128-1133. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||