化学学报 ›› 2024, Vol. 82 ›› Issue (2): 115-118.DOI: 10.6023/A23080384 上一篇 下一篇
所属专题: 有机氟化学合集
研究通讯
投稿日期:2023-08-19
发布日期:2023-11-24
基金资助:
Jian Zhenga,c, Jin-Hong Lina,b( ), Ji-Chang Xiaoa(
), Ji-Chang Xiaoa( )
)
Received:2023-08-19
Published:2023-11-24
Contact:
E-mail: Supported by:文章分享
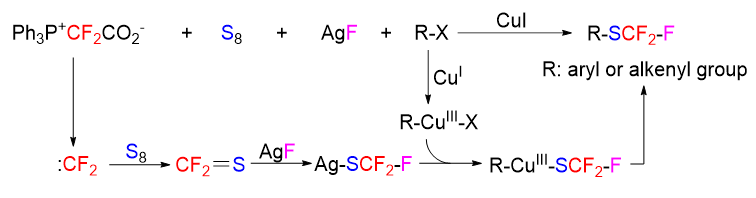
二氟卡宾在有机氟化物的合成中发挥了重要作用. 之前的发现, 二氟卡宾能与硫单质反应产生硫代氟光气, 这对二氟卡宾化学的新发现以及硫代氟光气的应用研究都具有重要价值. 利用这一路径已经实现了端基炔烃和烷基卤化物的三氟甲硫基化. 在此, 继续深入研究这一路径在合成上的应用, 并实现了芳基和烯基碘化物的三氟甲硫基化. 三氟甲硫基化是有机氟化学的一个重要研究方向, 常用方法一般需要使用含CF3S基团的昂贵试剂. 在该方法中, CF3S基团是由二氟卡宾、硫单质和氟离子现场产生的, 所用试剂都廉价易得.
郑剑, 林锦鸿, 肖吉昌. 基于二氟卡宾转化的芳基和烯基碘化物的三氟甲硫基化[J]. 化学学报, 2024, 82(2): 115-118.
Jian Zheng, Jin-Hong Lin, Ji-Chang Xiao. Difluorocarbene-based Trifluoromethylthiolation of Aryl and Alkenyl Iodides[J]. Acta Chimica Sinica, 2024, 82(2): 115-118.
| Entry | MF | Temp./℃ | Ratiob | Solv. | Yieldc/% |
|---|---|---|---|---|---|
| 1 | CsF | 100 | 1∶2∶4∶3∶0 | EtOAc | 0 |
| 2 | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 17 |
| 3d | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 0 |
| 4e | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 12 |
| 5f | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 3 |
| 6 | KF | 100 | 1∶2∶4∶3∶1 | EtOAc | 8 |
| 7 | TBAT | 100 | 1∶2∶4∶3∶1 | EtOAc | Trace |
| 8g | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 16 |
| 9h | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 0 |
| 10 | CsF | 100 | 1∶2∶4∶5∶1 | EtOAc | 20 |
| 11 | CsF | 100 | 1∶2∶6∶5∶1 | Dioxane | 36 |
| 12 | CsF | 80 | 1∶2∶6∶5∶1 | Dioxane | 11 |
| 13 | CsF | 110 | 1∶2∶6∶5∶1 | Dioxane | 37 |
| 14i | AgF | 110 | 1∶3∶9∶3∶3 | Dioxane | 64 |
| 15i | AgF | 110 | 1∶3∶9∶2.5∶3 | Dioxane | 78 |
| 16i,j | AgF | 110 | 1∶3∶9∶2.5∶3 | Dioxane | 87 |
| Entry | MF | Temp./℃ | Ratiob | Solv. | Yieldc/% |
|---|---|---|---|---|---|
| 1 | CsF | 100 | 1∶2∶4∶3∶0 | EtOAc | 0 |
| 2 | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 17 |
| 3d | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 0 |
| 4e | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 12 |
| 5f | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 3 |
| 6 | KF | 100 | 1∶2∶4∶3∶1 | EtOAc | 8 |
| 7 | TBAT | 100 | 1∶2∶4∶3∶1 | EtOAc | Trace |
| 8g | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 16 |
| 9h | CsF | 100 | 1∶2∶4∶3∶1 | EtOAc | 0 |
| 10 | CsF | 100 | 1∶2∶4∶5∶1 | EtOAc | 20 |
| 11 | CsF | 100 | 1∶2∶6∶5∶1 | Dioxane | 36 |
| 12 | CsF | 80 | 1∶2∶6∶5∶1 | Dioxane | 11 |
| 13 | CsF | 110 | 1∶2∶6∶5∶1 | Dioxane | 37 |
| 14i | AgF | 110 | 1∶3∶9∶3∶3 | Dioxane | 64 |
| 15i | AgF | 110 | 1∶3∶9∶2.5∶3 | Dioxane | 78 |
| 16i,j | AgF | 110 | 1∶3∶9∶2.5∶3 | Dioxane | 87 |
| [5] |
(i) Zhang, X.-Y.; Sun, S.-P.; Sang, Y.-Q.; Xue, X.-S.; Min, Q.-Q.; Zhang, X. Angew. Chem., Int. Ed. 2023, e202306501.
pmid: 31548670 |
| [6] |
(a) Kim, Y.; Heo, J.; Kim, D.; Chang, S.; Seo, S. Nat. Commun. 2020, 11, 4761.
doi: 10.1038/s41467-020-18557-8 |
|
(b) Su, J.; Ma, X.; Ou, Z.; Song, Q. ACS Cent. Sci. 2020, 6, 1819.
doi: 10.1021/acscentsci.0c00779 |
|
|
(c) Liu, X.; Sheng, H.; Zhou, Y.; Song, Q. Org. Lett. 2021, 23, 2543.
doi: 10.1021/acs.orglett.1c00467 |
|
|
(d) Liu, A.; Ni, C.; Xie, Q.; Hu, J. Angew. Chem., Int. Ed. 2022, 61, e202115467.
doi: 10.1002/anie.v61.8 |
|
|
(e) Wang, S.; Li, X.; Jin, S.; Liu, K.; Dong, C.; Su, J.; Song, Q. Org. Chem. Front. 2022, 9, 1282.
doi: 10.1039/D1QO01899J |
|
|
(f) Zhang, G.; Shi, Q.; Hou, M.; Yang, K.; Wang, S.; Wang, S.; Li, W.; Li, C.; Qiu, J.; Xu, H.; Zhou, L.; Wang, C.; Li, S.-J.; Lan, Y.; Song, Q. CCS Chem. 2022, 4, 1671.
doi: 10.31635/ccschem.021.202100979 |
|
|
(g) Chen, S.; Huang, H.; Li, X.; Ma, X.; Su, J.; Song, Q. Org. Lett. 2023, 25, 1178.
doi: 10.1021/acs.orglett.3c00150 |
|
| [7] |
(a) Ma, X.; Zhou, Y.; Song, Q. Org. Lett. 2018, 20, 4777.
doi: 10.1021/acs.orglett.8b01888 |
|
(b) Ma, X.; Mai, S.; Zhou, Y.; Cheng, G.-J.; Song, Q. Chem. Commun. 2018, 54, 8960.
doi: 10.1039/C8CC04298E |
|
|
(c) Si, Y.-X.; Zhu, P.-F.; Zhang, S.-L. Org. Lett. 2020, 22, 9086.
doi: 10.1021/acs.orglett.0c03472 |
|
|
(d) Jiang, B.-J.; Zhang, S.-L. Adv. Synth. Catal. 2022, 364, 2157.
doi: 10.1002/adsc.v364.13 |
|
| [8] |
(a) Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513.
doi: 10.1039/c3cc44271c pmid: 24114936 |
|
(b) Zheng, J.; Lin, J.-H.; Cai, J.; Xiao, J.-C. Chem.-Eur. J. 2013, 19, 15261.
doi: 10.1002/chem.201303248 pmid: 24114936 |
|
|
(c) Lin, J.-H.; Xiao, J.-C. Acc. Chem. Res. 2020, 53, 1498.
doi: 10.1021/acs.accounts.0c00244 pmid: 24114936 |
|
| [9] |
(a) Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A. J. Org. Chem. 2014, 79, 7122.
doi: 10.1021/jo501289v pmid: 26862998 |
|
(b) Liu, Y.; Zhang, K.; Huang, Y.; Pan, S.; Liu, X.-Q.; Yang, Y.; Jiang, Y.; Xu, X.-H. Chem. Commun. 2016, 52, 5969.
doi: 10.1039/C6CC00666C pmid: 26862998 |
|
|
(c) Panferova, L. I.; Tsymbal, A. V.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2016, 18, 996.
doi: 10.1021/acs.orglett.6b00117 pmid: 26862998 |
|
|
(d) Panferova, L. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Chem. Commun. 2019, 55, 1314.
doi: 10.1039/C8CC09115C pmid: 26862998 |
|
|
(e) Ilin, E. A.; Smirnov, V. O.; Volodin, A. D.; Korlyukov, A. A.; Dilman, A. D. Chem. Commun. 2020, 56, 7140.
doi: 10.1039/D0CC02567D pmid: 26862998 |
|
|
(f) Kee, C. W.; Tack, O.; Guibbal, F.; Wilson, T. C.; Isenegger, P. G.; Imiolek, M.; Verhoog, S.; Tilby, M.; Boscutti, G.; Ashworth, S.; Chupin, J.; Kashani, R.; Poh, A. W. J.; Sosabowski, J. K.; Macholl, S.; Plisson, C.; Cornelissen, B.; Willis, M. C.; Passchier, J.; Davis, B. G.; Gouverneur, V. J. Am. Chem. Soc. 2020, 142, 1180.
doi: 10.1021/jacs.9b11709 pmid: 26862998 |
|
|
(g) Jia, Y.; Yuan, Y.; Huang, J.; Jiang, Z.-X.; Yang, Z. Org. Lett. 2021, 23, 2670.
doi: 10.1021/acs.orglett.1c00577 pmid: 26862998 |
|
| [10] |
(a) Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236.
doi: 10.1002/anie.v54.45 |
|
(b) Zheng, J.; Cheng, R.; Lin, J.-H.; Yu, D. H.; Ma, L.; Jia, L.; Zhang, L.; Wang, L.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2017, 56, 3196.
doi: 10.1002/anie.v56.12 |
|
|
(c) Yu, J.; Lin, J.-H.; Xiao, J.-C. Angew. Chem., Int. Ed. 2017, 56, 16669.
doi: 10.1002/anie.v56.52 |
|
| [11] |
Yu, J.; Lin, J.-H.; Yu, D.; Du, R.; Xiao, J.-C. Nat. Commun. 2019, 10, 5362.
doi: 10.1038/s41467-019-13359-z |
| [12] |
Zhang, M.; Lin, J.-H.; Xiao, J.-C. Angew. Chem., Int. Ed. 2019, 58, 6079.
doi: 10.1002/anie.v58.18 |
| [13] |
(a) Middleton, W. J.; Howard, E. G.; Sharkey, W. H. J. Am. Chem. Soc. 1961, 83, 2589.
doi: 10.1021/ja01472a045 |
|
(b) Middleton, W. J.; Howard, E. G.; Sharkey, W. H. J. Org. Chem. 1965, 30, 1375.
doi: 10.1021/jo01016a008 |
|
|
(c) Eschwey, M.; Sundermeyer, W.; Stephenson, D. S. Chem. Ber. 1983, 116, 1623.
doi: 10.1002/cber.v116:4 |
|
|
(d) Waterfeld, A. Chem. Ber. 1990, 123, 1635.
doi: 10.1002/cber.v123:8 |
|
| [14] |
(a) Luo, J.-J.; Zhang, M.; Lin, J.-H.; Xiao, J.-C. J. Org. Chem. 2017, 82, 11206.
doi: 10.1021/acs.joc.7b01701 |
|
(b) Liu, Z.; Long, J.; Xiao, X.; Lin, J.-H.; Zheng, X.; Xiao, J.-C.; Cao, Y.-C. Chin. Chem. Lett. 2019, 30, 714.
doi: 10.1016/j.cclet.2018.11.013 |
|
| [1] |
(a) Brahms, D.; Dailey, W. Chem. Rev. 1996, 96, 1585.
pmid: 11848805 |
|
(b) Dilman, A. D.; Levin, V. V. Acc. Chem. Res. 2018, 51, 1272.
doi: 10.1021/acs.accounts.8b00079 pmid: 11848805 |
|
|
(c) Zhang, W.; Wang, Y. Tetrahedron Lett. 2018, 59, 1301.
pmid: 11848805 |
|
|
(d) Zhou, W.; Pan, W.-J.; Chen, J.; Zhang, M.; Lin, J.-H.; Cao, W.; Xiao, J.-C. Chem. Commun. 2021, 57, 9316.
doi: 10.1039/D1CC04029D pmid: 11848805 |
|
|
(e) Ma, X.; Su, J.; Song, Q. Acc. Chem. Res. 2023, 56, 592.
doi: 10.1021/acs.accounts.2c00830 pmid: 11848805 |
|
| [2] |
(a) Park, J. D.; Benning, A. F.; Downing, F. B.; Laucius, J. F.; McHarness, R. C. Ind. Eng. Chem. 1947, 39, 354.
doi: 10.1021/ie50447a624 pmid: 26636182 |
|
(b) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496.
doi: 10.1021/jacs.5b09888 pmid: 26636182 |
|
|
(c) Zheng, J.; Lin, J.-H.; Yu, L.-Y.; Wei, Y.; Zheng, X.; Xiao, J.-C. Org. Lett. 2015, 17, 6150.
doi: 10.1021/acs.orglett.5b03159 pmid: 26636182 |
|
|
(d) Zhang, Z.; Yu, W.; Wu, C.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2016, 55, 273.
doi: 10.1002/anie.v55.1 pmid: 26636182 |
|
|
(e) Li, L.; Ni, C.; Xie, Q.; Hu, M.; Wang, F.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 9971.
doi: 10.1002/anie.v56.33 pmid: 26636182 |
|
| [3] |
(a) Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390.
doi: 10.1002/anie.v52.47 |
|
(b) Deng, X.-Y.; Lin, J.-H.; Zheng, J.; Xiao, J.-C. Chem. Commun. 2015, 51, 8805.
doi: 10.1039/C5CC02736E |
|
|
(c) Xie, Q.; Ni, C.; Zhang, R.; Li, L.; Rong, J.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 3206.
doi: 10.1002/anie.v56.12 |
|
|
(d) Sap, J. B. I.; Meyer, C. F.; Ford, J.; Straathof, N. J. W.; Durr, A. B.; Lelos, M. J.; Paisey, S. J.; Mollner, T. A.; Hell, S. M.; Trabanco, A. A.; Genicot, C.; am Ende, C. W.; Paton, R. S.; Tredwell, M.; Gouverneur, V. Nature 2022, 606, 102.
doi: 10.1038/s41586-022-04669-2 |
|
| [4] |
(a) Fujioka, Y.; Amii, H. Org. Lett. 2008, 10, 769.
doi: 10.1021/ol702851t |
|
(b) Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H.; Jog, P.; Ganesh, S.; Prakash, G.; Olah, G. Angew. Chem., Int. Ed. 2011, 50, 7153.
doi: 10.1002/anie.v50.31 |
|
|
(c) Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411.
doi: 10.1039/C0CC04548A |
|
|
(d) Rulliere, P.; Cyr, P.; Charette, A. B. Org. Lett. 2016, 18, 1988.
doi: 10.1021/acs.orglett.6b00573 |
|
|
(e) Garcia-Dominguez, A.; West, T. H.; Primozic, J. J.; Grant, K. M.; Johnston, C. P.; Cumming, G. G.; Leach, A. G.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2020, 142, 14649.
doi: 10.1021/jacs.0c06751 |
|
| [5] |
(a) Brothers, P. J.; Roper, W. R. Chem. Rev. 1988, 88, 1293.
doi: 10.1021/cr00089a014 pmid: 31548670 |
|
(b) Harrison, D. J.; Lee, G. M.; Leclerc, M. C.; Korobkov, I.; Baker, R. T. J. Am. Chem. Soc. 2013, 135, 18296.
doi: 10.1021/ja411503c pmid: 31548670 |
|
|
(c) Deng, X.-Y.; Lin, J.-H.; Xiao, J.-C. Org. Lett. 2016, 18, 4384.
doi: 10.1021/acs.orglett.6b02141 pmid: 31548670 |
|
|
(d) Feng, Z.; Min, Q.-Q.; Zhang, X. Org. Lett. 2016, 18, 44.
doi: 10.1021/acs.orglett.5b03206 pmid: 31548670 |
|
|
(e) Feng, Z.; Min, Q. Q.; Fu, X. P.; An, L.; Zhang, X. Nat. Chem. 2017, 9, 918.
doi: 10.1038/nchem.2746 pmid: 31548670 |
|
|
(f) Fu, X.-P.; Xue, X.-S.; Zhang, X.-Y.; Xiao, Y.-L.; Zhang, S.; Guo, Y.-L.; Leng, X.; Houk, K. N.; Zhang, X. Nat. Chem. 2019, 11, 948.
doi: 10.1038/s41557-019-0331-9 pmid: 31548670 |
|
|
(g) Xu, Z.-W.; Zhang, W.; Lin, J.-H.; Jin, C. M.; Xiao, J.-C. Chin. J. Chem. 2020, 38, 1647.
doi: 10.1002/cjoc.v38.12 pmid: 31548670 |
|
|
(h) Zeng, X.; Li, Y.; Min, Q.-Q.; Xue, X.-S.; Zhang, X. Nat. Chem. 2023.
pmid: 31548670 |
|
| [14] |
(c) He, G.; Jiang, Y.-H.; Xiao, X.; Lin, J.-H.; Zheng, X.; Du, R.-B.; Cao, Y.-C.; Xiao, J.-C. J. Fluorine Chem. 2020, 230, 109437.
doi: 10.1016/j.jfluchem.2019.109437 |
| [15] |
(a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525.
doi: 10.1021/cr60274a001 pmid: 4747963 |
|
(b) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207.
pmid: 4747963 |
|
|
(c) Hansch, C.; Leo, A.; Taft, R. Chem. Rev. 1991, 91, 165.
doi: 10.1021/cr00002a004 pmid: 4747963 |
|
| [16] |
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
doi: 10.1021/cr500193b |
| [17] |
(a) Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513.
doi: 10.1021/ar4003202 |
|
(b) Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227.
doi: 10.1021/acs.accounts.5b00047 |
|
|
(c) Xu, C.; Wang, S.; Shen, Q. ACS Sustainable Chem. Eng. 2022, 10, 6889.
doi: 10.1021/acssuschemeng.2c01006 |
|
|
(d) Shen, Q. J. Org. Chem. 2023, 88, 3359.
doi: 10.1021/acs.joc.2c02777 |
|
|
(e) Qing, F.-L.; Liu, X.-Y.; Ma, J.-A.; Shen, Q.; Song, Q.; Tang, P. CCS Chem. 2022, 4, 2518.
doi: 10.31635/ccschem.022.202201935 |
| [1] | 李树森, 王剑波. 不对称三氟甲硫基化反应研究进展[J]. 化学学报, 2018, 76(12): 913-924. |
| [2] | 马星星, 轩晴晴, 宋秋玲. 含氮杂环的N—H和O—H二氟甲基化反应[J]. 化学学报, 2018, 76(12): 972-976. |
| [3] | 张盼盼, 吕龙, 沈其龙. 直接三氟甲硫基化试剂及方法的研究进展[J]. 化学学报, 2017, 75(8): 744-769. |
| [4] | 徐佳斌, 陈品红, 叶金星, 刘国生. 钯催化的芳基C-H键三氟甲硫基化反应[J]. 化学学报, 2015, 73(12): 1294-1297. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||