化学学报 ›› 2024, Vol. 82 ›› Issue (7): 763-771.DOI: 10.6023/A24040147 上一篇 下一篇
研究论文
投稿日期:2024-04-28
发布日期:2024-06-12
基金资助:
Chengjun Wang, Yueliang Wang, Huiqiao Wang, Zhaoxiang Deng*( )
)
Received:2024-04-28
Published:2024-06-12
Contact:
*E-mail: Supported by:文章分享
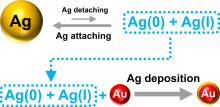
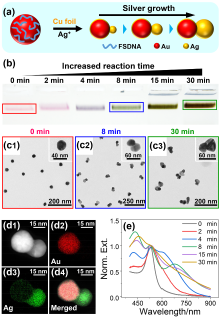
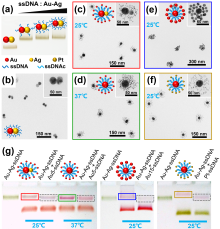
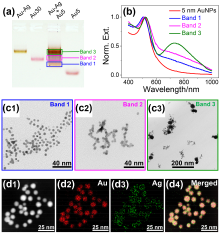
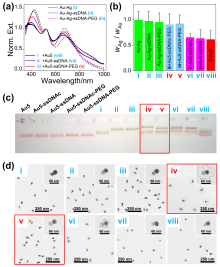
通过自组装构筑不同金属纳米粒子间的异质组装体, 对于理解和调控等离激元近场耦合, 以及“点亮”等离激元光学暗态等具有重要意义. 利用组装体中不同金属的催化活性差异, 还可望实现协同或接力催化. 金和银纳米材料在可见光区具有强吸收和散射, 成为等离激元器件的常见选择. 由于银的化学性质较金活泼, 其胶体稳定性也不及金纳米粒子, 实际应用中远没有金纳米粒子普及. 与金相比, 银纳米粒子的带间跃迁与等离激元共振较为远离, 其等离激元损耗也更低, 呈现比金更强且更对称的等离激元共振峰, 展现出诱人的光学性质. 在自组装结构中同时引入金和银两种纳米砌块, 可望实现同质结构无法获得的功能. 然而, 由于两者具有显著不同的化学性质, 需对其化学相容性进行探究. 本工作首先制备出具有电荷转移等离激元共振特性的金-银纳米粒子, 其独特的双金属结构和良好的胶体稳定性不仅方便其DNA功能化和可编程自组装, 同时也有利于对金粒子表面沉积的银纳米粒子尺寸和形貌变化进行观测. 基于Au-Ag纳米粒子与金纳米粒子的简单混合和组装实验, 清楚揭示了金纳米粒子增强的银刻蚀现象, 并提出金表面促进银转移反应(GSPSTR)这一化学机制, 以及增强银和金纳米单元间化学相容性的有效办法. 本工作对于利用DNA导向组装实现银纳米粒子参与的多元金属异质结构, 以及发展等离激元和催化等功能与应用具有重要意义, 更为正确理解金属银参与的纳米合成与组装等过程提供了新认识.
王程鋆, 王悦靓, 王会巧, 邓兆祥. 金-银纳米异质组装过程的化学不相容性[J]. 化学学报, 2024, 82(7): 763-771.
Chengjun Wang, Yueliang Wang, Huiqiao Wang, Zhaoxiang Deng. Unveiling the Chemical Incompatibility of Au-Ag Heteronanoassembly[J]. Acta Chimica Sinica, 2024, 82(7): 763-771.





| Metal content | R1 | R2 | S | Total |
|---|---|---|---|---|
| mAu/μg | 2.928 | 7.014 | 0.162 | 10.104 |
| mAg/μg | 0.492 | 0.978 | 0.012 | 1.482 |
| Metal content | R1 | R2 | S | Total |
|---|---|---|---|---|
| mAu/μg | 2.928 | 7.014 | 0.162 | 10.104 |
| mAg/μg | 0.492 | 0.978 | 0.012 | 1.482 |


| [1] |
Kelly K. L.; Coronado E.; Zhao L. L.; Schatz G. C. J. Phys. Chem. B 2003, 107, 668.
|
| [2] |
Kreibig U.; Vollmer M. Optical properties of metal clusters, Springer, Berlin, 1995.
|
| [3] |
Barnes W. L.; Dereux A.; Ebbesen T. W. Nature 2003, 424, 824.
|
| [4] |
Yang X.; Zhang Z.; Su M.; Song Y. Acta Chim. Sinica 2022, 80, 80 (in Chinese).
doi: 10.6023/A21100469 |
|
(杨旭, 张泽英, 苏萌, 宋延林, 化学学报, 2022, 80, 80.)
doi: 10.6023/A21100469 |
|
| [5] |
Dong Y. Y.; Hu C. Y.; Xiong H. L.; Long R.; Xiong Y. J. ACS Catal. 2023, 13, 6730.
|
| [6] |
Aslam U.; Rao V. G.; Chavez S.; Linic S. Nat. Catal. 2018, 1, 656.
|
| [7] |
Jackman J. A.; Rahim Ferhan A.; Cho N.-J. Chem. Soc. Rev. 2017, 46, 3615.
doi: 10.1039/c6cs00494f pmid: 28383083 |
| [8] |
Masson J.-F. ACS Sens. 2017, 2, 16.
|
| [9] |
Tokel O.; Inci F.; Demirci U. Chem. Rev. 2014, 114, 5728.
doi: 10.1021/cr4000623 pmid: 24745365 |
| [10] |
Hang Y.; Wang A.; Wu N. Chem. Soc. Rev. 2024, 53, 2932.
|
| [11] |
Wy Y.; Jung H.; Hong J. W.; Han S. W. Acc. Chem. Res. 2022, 55, 831.
|
| [12] |
Prodan E.; Radloff C.; Halas N. J.; Nordlander P. Science 2003, 302, 419.
pmid: 14564001 |
| [13] |
Romero I.; Aizpurua J.; Bryant G. W.; García de Abajo F. J. Opt. Express 2006, 14, 9988.
|
| [14] |
Zhu W.; Esteban R.; Borisov A. G.; Baumberg J. J.; Nordlander P.; Lezec H. J.; Aizpurua J.; Crozier K. B. Nat. Commun. 2016, 7, 11495.
|
| [15] |
Ma J.; Huang L.; Zhou B.; Yao L. Acta Chim. Sinica 2022, 80, 1507 (in Chinese).
|
|
(马进越, 黄露霏, 周宝文, 姚琳, 化学学报, 2022, 80, 1507.)
doi: 10.6023/A22070308 |
|
| [16] |
Yang Q.; Liu X.; Wang C.; Xu M.; Yao J. Acta Chim. Sinica 2024, 82, 281 (in Chinese).
|
|
(杨青, 刘肖宇, 王晨, 徐敏敏, 姚建林, 化学学报, 2024, 82, 281.)
doi: 10.6023/A23120527 |
|
| [17] |
Brown L. V.; Sobhani H.; Lassiter J. B.; Nordlander P.; Halas N. J. ACS Nano 2010, 4, 819.
doi: 10.1021/nn9017312 pmid: 20092361 |
| [18] |
Deng T.-S.; Parker J.; Yifat Y.; Shepherd N.; Scherer N. F. J. Phys. Chem. C 2018, 122, 27662.
|
| [19] |
Sheikholeslami S.; Jun Y. W.; Jain P. K.; Alivisatos A. P. Nano Lett. 2010, 10, 2655.
doi: 10.1021/nl101380f pmid: 20536212 |
| [20] |
Yamada Y.; Tsung C.-K.; Huang W.; Huo Z.; Habas S. E.; Soejima T.; Aliaga C. E.; Somorjai G. A.; Yang P. D. Nat. Chem. 2011, 3, 372.
|
| [21] |
Yan H.; He K.; Samek I. A.; Jing D.; Nanda M. G.; Stair P. C.; Notestein J. M. Science 2021, 371, 1257.
|
| [22] |
Huang J.; Mensi M.; Oveisi E.; Mantella V.; Buonsanti R. J. Am. Chem. Soc. 2019, 141, 2490.
|
| [23] |
Liu M. Z.; Guyot-Sionnest P. J. Phys. Chem. B 2004, 108, 5882.
|
| [24] |
Linic S.; Christopher P.; Ingram D. B. Nat. Mater. 2011, 10, 911.
|
| [25] |
Wang F.; Shen Y. R. Phys. Rev. Lett. 2006, 97, 206806.
|
| [26] |
Blaber M. G.; Henry A.-I.; Bingham J. M.; Schatz G. C.; Van Duyne R. P. J. Phys. Chem. C 2012, 116, 393.
|
| [27] |
Singh K. R. B.; Natarajan A.; Pandey S. S. ACS Appl. Bio Mater. 2023, 6, 4549.
|
| [28] |
Lee K. S.; El-Sayed M. A. J. Phys. Chem. B 2006, 110, 19220.
|
| [29] |
Hill C. M.; Pan S. J. Am. Chem. Soc. 2013, 135, 17250.
|
| [30] |
Zhu P.; Lou C.; Shi Y.; Wang C. Acta Chim. Sinica 2022, 80, 1385 (in Chinese).
|
|
(朱鹏飞, 娄晨思, 史雨翰, 王传义, 化学学报, 2022, 80, 1385.)
doi: 10.6023/A22060266 |
|
| [31] |
Jiang X.; Zeng Q.; Yu A. Langmuir 2007, 23, 2218.
|
| [32] |
Chen Y.; Wang C. G.; Ma Z. F.; Su Z. M. Nanotechnology 2007, 18, 325602.
|
| [33] |
An J.; Tang B.; Zheng X. L.; Zhou J.; Dong F. X.; Xu S. P.; Wang Y.; Zhao B.; Xu W. Q. J. Phys. Chem. C 2008, 112, 15176.
|
| [34] |
Xue J.; Jia Y.; Yang L.; Feng J.; Wu D.; Ren X.; Du Y.; Ju H.; Wei Q. Anal. Chem. 2020, 92, 14203.
|
| [35] |
Zhang Q.; Ge J.; Pham T.; Goebl J.; Hu Y.; Lu Z.; Yin Y. Angew. Chem. Int. Ed. 2009, 48, 3516.
doi: 10.1002/anie.200900545 pmid: 19347914 |
| [36] |
Sun Y. G.; Mayers B.; Xia Y. N. Nano Lett. 2003, 3, 675.
|
| [37] |
Tang B.; An J.; Zheng X. L.; Xu S. P.; Li D. M.; Zhou J.; Zhao B.; Xu W. Q. J. Phys. Chem. C 2008, 112, 18361.
|
| [38] |
Hu S.; Yi T.; Huang Z.; Liu B.; Wang J.; Yi X.; Liu J. Mater. Horiz. 2019, 6, 155.
|
| [39] |
Chen N.; Wang Y. L.; Song X. J.; Li Y. J.; Deng Z. X. Nano Lett. 2022, 22, 8550.
|
| [40] |
Wen F.; Zhang Y.; Gottheim S.; King N. S.; Zhang Y.; Nordlander P.; Halas N. J. ACS Nano 2015, 9, 6428.
|
| [41] |
Fang L. L.; Liu D. L.; Wang Y. L.; Li Y. J.; Song L.; Gong M.; Li Y.; Deng Z. X. Nano Lett. 2018, 18, 7014.
|
| [42] |
Zhu Q. Q.; Song X. J.; Deng Z. X. Acta Chim. Sinica 2020, 78, 675 (in Chinese).
|
|
(朱青青, 宋晓君, 邓兆祥, 化学学报, 2020, 78, 675.)
doi: 10.6023/A20050145 |
|
| [43] |
Liu M.; Fang L. L.; Li Y. L.; Gong M.; Xu A.; Deng Z. X. Chem. Sci. 2016, 7, 5435.
|
| [44] |
Hao Y.; Li Y. J.; Song L.; Deng Z. X. J. Am. Chem. Soc. 2021, 143, 3065.
|
| [45] |
Ye Y. C.; Hao Y.; Ye M. Y.; Song X. J.; Deng Z. X. Small 2022, 18, 2202458.
|
| [46] |
Pal S.; Sharma J.; Yan H.; Liu Y. Chem. Commun. 2009, 6059.
|
| [47] |
Liu H.; Qu J.; Chen Y.; Li J.; Ye F.; Lee J. Y.; Yang J. J. Am. Chem. Soc. 2012, 134, 11602.
|
| [48] |
Xue C.; Métraux G. S.; Millstone J. E.; Mirkin C. A. J. Am. Chem. Soc. 2008, 130, 8337.
|
| [49] |
Wu X. M.; Redmond P. L.; Liu H. T.; Chen Y. H.; Steigerwald M.; Brus L. J. Am. Chem. Soc. 2008, 130, 9500.
|
| [50] |
Wu Z. Angew. Chem., Int. Ed. 2012, 51, 2934.
|
| [51] |
Sahu P.; Prasad B. L. V. Colloids Surf. A 2015, 478, 30.
|
| [52] |
Peng J.; Huang B.; Wang P.; Pei Y. J. Phys. Chem. A 2022, 126, 8910.
|
| [53] |
Gan Z.; Xia N.; Wu Z. Acc. Chem. Res. 2018, 51, 2774.
|
| [54] |
Liu X.; Astruc D. Adv. Mater. 2017, 29, 1605305.
|
| [55] |
Jin F.; Dong H.; Zhao Y.; Zhuang S.; Liao L.; Yan N.; Gu W.; Zha J.; Yuan J.; Li J.; Deng H.; Gan Z.; Yang J.; Wu Z. Acta Chim. Sinica 2020, 78, 407 (in Chinese).
|
|
(金凤鸣, 董宏伟, 赵燕, 庄胜利, 廖玲文, 闫楠, 古万苗, 查珺, 袁金云, 李进, 邓海腾, 甘自保, 杨金龙, 伍志鲲, 化学学报, 2020, 78, 407.)
doi: 10.6023/A20040134 |
|
| [56] |
Lei P. C.; Li Y. J.; Song X. J.; Hao Y.; Deng Z. X. Angew. Chem. Int. Ed. 2022, 61, e202203568.
|
| [57] |
Ye M. Y.; Song L.; Ye Y. C.; Deng Z. X. J. Am. Chem. Soc. 2023, 145, 25653.
|
| [58] |
Li Y. L.; Deng Z. X. Acc. Chem. Res. 2019, 52, 3442.
|
| [59] |
Herrero E.; Buller L. J.; Abruña H. D. Chem. Rev. 2001, 101, 1897.
pmid: 11710235 |
| [60] |
Chirea M.; Collins S. S.; Wei X.; Mulvaney P. J. Phys. Chem. Lett. 2014, 5, 4331.
|
| [61] |
Hong M.; Yokota Y.; Wong R. A.; Hayazawa N.; Kazuma E.; Kim Y. J. Phys. Chem. C 2021, 125, 16569.
|
| [62] |
Wang H.; Li Y.; Gong M.; Deng Z. Chem. Sci. 2014, 5, 1015.
|
| [1] | 郭宜君, 魏冰, 周翔, 姚东宝, 梁好均. DNA步行器调控的纳米粒子超晶格[J]. 化学学报, 2021, 79(2): 192-199. |
| [2] | 袁宏宇, 徐敏敏, 姚建林. 电化学SPR协同催化对氯苯硫酚界面反应的SERS研究[J]. 化学学报, 2021, 79(12): 1481-1485. |
| [3] | 杨晶亮, 杨伟民, 林嘉盛, 汪安, 徐娟, 李剑锋. 电场强度对等离激元诱导热电子的影响[J]. 化学学报, 2020, 78(7): 670-674. |
| [4] | 左方涛, 徐威, 赵爱武. 基于功能化Fe3O4@Ag纳米粒子快速检测Hg2+的SERS方法[J]. 化学学报, 2019, 77(4): 379-386. |
| [5] | 张晨杰, 张婧, 林洁茹, 金琦, 徐敏敏, 姚建林. 金纳米粒子单层膜表面SPR催化反应的原位SERS研究[J]. 化学学报, 2017, 75(9): 860-865. |
| [6] | 王鑫, 谭丽丽, 杨英威. 金粒子包封介孔二氧化硅杂化载药控释体系[J]. 化学学报, 2016, 74(4): 303-311. |
| [7] | 范艳斌, 陈道勇. 寡聚(4-乙烯基苯基磷酸)/金纳米粒子的磷酸酶可视化检测研究[J]. 化学学报, 2014, 72(9): 1012-1016. |
| [8] | 赵刘斌, 黄逸凡, 吴德印, 任斌. 对氨基苯硫酚分子的表面增强拉曼光谱及等离激元光催化反应[J]. 化学学报, 2014, 72(11): 1125-1138. |
| [9] | 张召香, 张飞, 刘营. 基于场放大进样及Au纳米粒子双重富集用于大肠杆菌检测的毛细管电泳电化学免疫分析法[J]. 化学学报, 2012, 70(21): 2251-2256. |
| [10] | 李迎, 林钊, 李蓉卓, 刘霞. 纳米金增强SPR 检测产毒赭曲霉中PKS 基因的特异性碱基[J]. 化学学报, 2012, 70(11): 1304-1308. |
| [11] | 曾国平, 向东山, 李丽, 何治柯. 金纳米粒子作探针共振瑞利散射光谱法测定牛奶中三聚氰胺[J]. 化学学报, 2011, 69(23): 2859-2864. |
| [12] | 张轶, 梁爱惠, 周莲平, 覃惠敏, 欧阳辉祥, 王鹏飞, 蒋治良. 双链DNA裂解-纳米金共振散射光谱探针检测痕量 UO22+ [J]. 化学学报, 2011, 69(18): 2153-2158. |
| [13] | 杨晶, 王著元, 张若虎, 宋春元, 李锦, 高文成, 崔一平. 基于金纳米聚集体的核壳型SERS探针及其细胞内应用[J]. 化学学报, 2011, 69(16): 1890-1894. |
| [14] | 陈晓惠, 杜建修. 金纳米粒子催化鲁米诺-异烟肼化学发光反应及其分析应用[J]. 化学学报, 2011, 69(06): 745-751. |
| [15] | 赵三平, 曹孟杰, 李利燕, 徐卫林. 金纳米粒子杂化超分子水凝胶的制备与性能[J]. 化学学报, 2011, 69(04): 492-496. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||