

基于荧光偏振的高灵敏度蛋白激酶活性分析
收稿日期: 2013-09-28
网络出版日期: 2013-10-23
基金资助
项目受国家自然科学基金(No. 91127035)和教育部博士点基金(No. 20111301130001)资助.
Ultrasensitive Detection of Protein Kinase Activity by Using the Fluorescence Polarization Technique
Received date: 2013-09-28
Online published: 2013-10-23
Supported by
Project supported by the National Natural Science Foundation of China (No. 91127035) and Doctoral Fund of Ministry of Education of China (No. 20111301130001).
王志彬 , 张学晶 , 王愈聪 , 高金鹏 , 李正平 . 基于荧光偏振的高灵敏度蛋白激酶活性分析[J]. 化学学报, 2013 , 71(12) : 1620 -1624 . DOI: 10.6023/A13091013
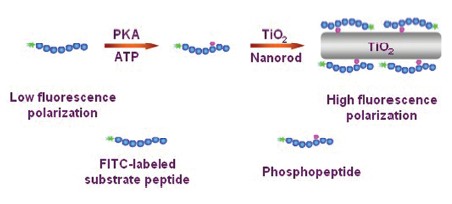
Protein phosphorylation by protein kinase plays a critical role in the process of cellular signal transduction, which is closely associated with many fundamental biological processes including cell growth and apoptosis. Aberrant states of protein kinase activities are involved in many diseases, such as diabetes, Alzheimer's disease and cancers. Moreover, protein kinase has now become one of the most important groups of drug targets. The screening of protein kinase inhibitors is becoming increasingly important in the targeted therapy. Therefore, rapid and sensitive detection of protein kinase activity is of great significance for better understanding the life process, clinical diagnosis as well as discovery of new targeted drugs. In this study, a simple, ultrasensitive and cost-effective assay is developed for detection of protein kinase activity by using fluorescence polarization technique based on the specific recognition ability of TiO2 nanorods for kinase-induced fluorescent phosphopeptides. In the presence of protein kinase, the fluorescence-labeled substrate peptides can be phosphorylated with the catalysis of the protein kinase. Then the phosphopeptides can specifically bind to Ti4+ on the surface of the TiO2 nanorods by means of phosphate groups, leading to remarkable change of rotation speed of the fluorophores, which subsequently results in the great increase of fluorescence polarization signal. By monitoring the fluorescence polarization signals, protein kinase A(PKA), a proof-of-concept kinase target, can be detected in the range of 0.0005~1.0 U·μL-1 and the detection limit is estimated to be 0.0004 U·μL-1, indicating that the proposed assay is one of the most sensitive assays for PKA activity detection. Furthermore, the proposed assay is also sucessfully applied to PKA inhibition assay by using H-89 as the model inhibitor of PKA. The IC50 value (inhibitor concentration producing 50% inhibition) is determined as 135 nmol·L-1, which is well consistent with that reported in the literatures. For the proposed assay, the TiO2 nanorods are commercially available with low cost and can be used without any modification. The fluorescence polarization can be directly measured in the homogeneous solution. Therefore, the new strategy provides a simple detection procedure, easy readout and cost-effective manner for protein kinase assay.
[1] Hunter, T. Cell 2000, 100, 113.
[2] Franklin, R. A.; McCubrey, J. A. Leukemia 2000, 14, 2019.
[3] Houseman, B. T.; Huh, J. H.; Kron, S. J.; Mrksich, M. Nat. Biotechnol. 2002, 20, 270.
[4] Li, Y. J.; Xie, W. H.; Fang, G. J. Anal. Bioanal. Chem. 2008, 390, 2049.
[5] Wang, C. L.; Wei, L. Y.; Yuan, C. J.; Hwang, K. C. Anal. Chem. 2012, 84, 971.
[6] Xu, S. J.; Liu, Y.; Wang, T. H.; Li, J. H. Anal. Chem. 2010, 82, 9566.
[7] Yu, Y. H.; Anjum, R.; Kubota, K.; Rush, J.; Villen, J.; Gygi, S. P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 11606.
[8] Yoshida, T.; Sato, M.; Ozawa, T.; Umezawa, Y. Anal. Chem. 2000, 72, 6.
[9] Wu, J. J.; Yarwood, D. R.; Pham, Q.; Sills, M. A. J. Biomol. Screening 2000, 5, 23.
[10] Gaudet, E. A.; Huang, K. S.; Zhang, Y.; Huang, W.; Mark, D.; Sportsman, J. R. J. Biomol. Screening 2003, 8, 164.
[11] Perrin, F. J. Phys. Radium. 1926, 7, 390.
[12] Ji, J.; Yang, H.; Liu, Y.; Chen, H.; Kong, J. L.; Liu, B. H. Chem. Commun. 2009, 1508.
[13] Bai, J.; Zhao, Y. J.; Wang, Z. B.; Liu, C. H.; Wang, Y. C.; Li, Z. P. Anal. Chem. 2013, 85, 4813.
[14] Wang, Y.; Long, Y.-Q. Chin. J. Org. Chem. 2011, 31, 1595. (王勇, 龙亚秋, 有机化学, 2011, 31, 1595.)
[15] Marunaka, Y.; Niisato, N. Biochem. Pharmacol. 2003, 66, 1083.
[16] Davies, S. P.; Reddy, H.; Caivano, M.; Cohen, P. Biochem. J. 2000, 351, 95.
[17] Sazonova, O. V.; Blishchenko, E. Y.; Tolmazova, A. G.; Khachin, D. P.; Leontiev, K. V.; Karelin, A. A.; Ivanov, V. T. FEBS J. 2007, 274, 474.
/
| 〈 |
|
〉 |