

基于有机二硫化物氧化还原电对的非碘系染料敏化太阳能电池
收稿日期: 2014-09-24
网络出版日期: 2014-11-21
基金资助
项目受国家自然科学基金(Nos. 61177020, 11121091)资助.
A Novel Organic Disulfide/Thiolate Redox Mediator for Iodine-free Dye-sensitized Solar Cells
Received date: 2014-09-24
Online published: 2014-11-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 61177020, 11121091).
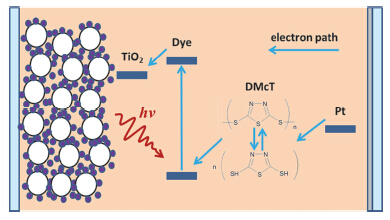
相比较于硅太阳能电池而言, 染料敏化太阳能电池具有成本低廉、制备简单的特点. 在目前研究中, 普遍采用碘离子作为氧化还原电对, 然而碘元素本身具有腐蚀性, 长期使用会腐蚀对电极从而影响器件寿命, 而且此类电解液在可见光区内有很强的吸收, 也不利于光能向电能的转换. 本文采用了一种新型有机二硫化物, 2,5-二硫基-1,3,4-噻二唑, 作为氧化还原介质, 其不仅能够利用其自身自均聚/自解聚反应来实现光电转换过程, 降低对电极的腐蚀, 而且在可见光区内的吸收很低, 也有利于制作背入射式的染料敏化太阳能电池体系. 通过器件的优化, 可以分别实现1.6% (100 mW·cm-2)和2.6% (13 mW·cm-2)的光电转换效率.
马英壮 , 郑灵灵 , 张立培 , 陈志坚 , 王树峰 , 曲波 , 肖立新 , 龚旗煌 . 基于有机二硫化物氧化还原电对的非碘系染料敏化太阳能电池[J]. 化学学报, 2015 , 73(3) : 257 -260 . DOI: 10.6023/A14090659
Over the last 20 years, much attention has been paid to renewable energy technology. Photovoltaic is a promising alternative to conventional fossil fuels. Dye-sensitized solar cells (DSCs) attract notable interest, not only due to their high efficiency and environmentally friendly nature, but also their easy fabrication and relatively low manufacture costs. Despite the high efficiencies, iodine/triiodine electrolytes have some disadvantages, such as the corrosion of the metallic electrodes and the sealing materials. It also absorbs visible light around 430 nm. Therefore, it is important to exploit the iodine-free redox couple in DSCs. An organic disulfide material of 2,5-dimercapto-1,3,4-thiadiazole (DMcT) is proved here to reduce and oxidize independently via homopolymerization and depolymerization. DMcT has been applied as cathode active material for lithium rechargeable batteries. Meanwhile, the self-redox property could be used as redox mediator in lieu of iodine/triiodine electrolytes. DMcT can be oxidized by self-polymerizing into PDMcT, which can be reduced by depolymerizing back to DMcT. In contrast to the conventional redox couples consisted of two different materials, DMcT can independently act as the redox mediator, which is the main difference between DMcT and the redox couples reported previously. Dye-sensitized solar cells consist of mesoporous TiO2, N719 dye, and this novel electrolyte achieved power conversion efficiency of 1.6% under 100 mW·cm-2 simulated sunlight (AM 1.5G) and a higher efficiency of 2.6% at weak illumination (13 mW·cm-2), implying its promising application prospect. Although the conversion efficiency is relatively low to the iodine/triiodine-based DSCs, this novel single self-redox mediator provides a new promising way to the iodine-free dye-sensitized solar cells.

Key words: dye-sensitized; solar cell; organic disulfide; redox couple
[1] O'Regan, B.; Grätzel, M. Nature 1991, 353, 737.
[2] Bach, U.; Lupo, D.; Comte, P.; Moser, J. E.; Weissortel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Nature 1998, 395, 583.
[3] Grätzel, M. Nature 2001, 414, 338.
[4] Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Chem. Rev. 2010, 110, 6595.
[5] Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Y. Jpn. J. Appl. Phys. 2006, 45, 638.
[6] Yuan, Q.; Chen, X. J.; Wang, J. T.; Zhai, J. Acta Chim. Sinica 2014, 72, 624. (苑琪, 陈雪景, 王京涛, 翟锦, 化学学报, 2014, 72, 624.)
[7] Cao, Z. C.; He, Z.; Deng, L. J.; Tan, S. T. Chin. J. Org. Chem. 2014, 34, 340. (曹镇财, 何舟, 邓利军, 谭松庭, 有机化学, 2014, 34, 340.)
[8] Wang, J. W.; Li, Y.; Xu, Y. L.; Li, Y.; Shen, K. H. Acta Chim. Sinica 2012, 70, 1278. (王纪伟, 李莹, 徐艳玲, 李杨, 申凯华, 化学学报, 2012, 70, 1278.)
[9] He, J. J.; Chen, S. X.; Wang, T. T.; Zeng, H. P. Chin. J. Org. Chem. 2012, 32, 472. (何俊杰, 陈舒欣, 王婷婷, 曾和平, 有机化学, 2012, 32, 472.)
[10] Yum, J. H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J. E.; Yi, C.; Nazeeruddin, M. K.; Grätzel, M. Nat. Commun. 2012, 3, 631.
[11] Yella, A.; Lee, H. W.; Tsao, H. N.; Yi, C.; Chandiran, A. K.; Nazeeruddin, M. K.; Diau, E. W.; Yeh, C. Y.; Zakeeruddin, S. M.; Grätzel, M. Science 2011, 334, 629.
[12] Boschloo, G.; Hagfeldt, A. Acc. Chem. Res. 2009, 42, 1819.
[13] Wang, M. K.; Chamberland, N.; Breau, L.; Moser, J. E.; Humphry-Baker, R.; Marsan, B.; Zakeeruddin, S. M.; Grätzel, M. Nat. Chem. 2010, 2, 385.
[14] Tian, H. N.; Jiang, X. A.; Yu, Z.; Kloo, L.; Hagfeldt, A.; Sun, L. Angew. Chem., Int. Ed. 2010, 49, 7328.
[15] Li, D.; Li, H.; Luo, Y.; Li, K.; Meng, Q.; Armand, M.; Chen, L. Adv. Funct. Mater. 2010, 20, 3358.
[16] Tian, H. N.; Yu, Z.; Hagfeldt, A.; Kloo, L.; Sun, L. J. Am. Chem. Soc. 2011, 133, 9413.
[17] Daeneke, T.; Kwon, T. H.; Holmes, A. B.; Duffy, N. W.; Bach, U.; Spiccia, L. Nat. Chem. 2011, 3, 211.
[18] Bai, Y.; Yu, Q. J.; Cai, N.; Wang, Y. H.; Zhang, M.; Wang, P. Chem. Commun. 2011, 47, 4376.
[19] Liu, M.; Visco, S.; De Jonghe, L. C. J. Electrochem. Soc. 1991, 138, 1896.
[20] Oyama, N.; Tatsuma, T.; Sato, T.; Sotomura, T. Nature 1995, 373, 598.
[21] Xiao, L. X.; Hou, T. L.; Guo, B. K.; Oyama, N. J. Central South Univ. (Sci. Technol.) 2004, 35, 774. (肖立新, 侯桃丽, 郭炳焜, 小山昇, 中南大学学报(自然科学版), 2004, 35, 774.)
[22] Wang, L.; Wu, M.; Gao, Y.; Ma, T. Appl. Phys. Lett. 2011, 98, 221102.
[23] Wu, H.; Lv, Z.; Chu, Z.; Wang, D.; Hou, S.; Zou, D. J. Mater. Chem. 2011, 21, 14815.
[24] Wang, M.; Moon, S. J.; Zhou, D.; Le Formal, F.; Cevey-Ha, N. L.; Humphry-Baker, R.; Grätzel, C.; Wang, P.; Zakeeruddin, S. M.; Grätzel, M. Adv. Funct. Mater. 2010, 20, 1821.
[25] Wang, Q.; Moser, J.-E.; Grätzel, M. J. Phys. Chem. B 2005, 109, 14945.
[26] Fabregat-Santiago, F.; Bisquert, J.; Cevey, L.; Chen, P.; Wang, M.; Zakeeruddin, S. M.; Grätzel, M. J. Am. Chem. Soc. 2009, 131, 558.
[27] Fabregat-Santiago, F.; Bisquert, J.; Palomares, E.; Otero, L.; Kuang, D.; Zakeeruddin, S. M.; Grätzel, M. J. Phys. Chem. C 2007, 111, 6550.
[28] Koh, J. K.; Kim, J.; Kim, B.; Kim, J. H.; Kim, E. Adv. Mater. 2011, 23, 1641.
[29] Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Sol. Energy Mater. Sol. Cells 2005, 87, 117.
/
| 〈 |
|
〉 |